Taletrectinib (Ibtrozi) 2025 Updates: Uses in Cancer, Side effects, Dosage, Expectations and more
Taletrectinib is an oral, next-generation tyrosine kinase inhibitor (TKI) designed to selectively target ROS1 and NTRK gene fusions. Developed to address resistance mutations and improve tolerability over earlier TKIs, it is currently being evaluated in clinical trials across multiple cancer types. Early results suggest promising activity, particularly in patients with fusion-positive tumors. This article will explore the uses of Taletrectinib in different types of cancer, its potential side effects, recommended dosage, and what patients can expect during treatment.
Which company produced Taletrectinib?
Taletrectinib, marketed under the brand name Ibtrozi in the United States, was originally discovered by Daiichi Sankyo, a global pharmaceutical company based in Japan. In 2018, the development rights for Taletrectinib were licensed to AnHeart Therapeutics, a clinical-stage biotech company focused on precision oncology. AnHeart advanced the development of Taletrectinib, particularly in ROS1-positive non-small cell lung cancer (NSCLC).
In March 2024, Nuvation Bio, a U.S.-based oncology company, announced the acquisition of AnHeart Therapeutics in an all-stock transaction. This gave Nuvation full global rights to Taletrectinib outside of previously licensed territories.
In Greater China, Innovent Biologics holds exclusive development and commercialization rights for Taletrectinib. The company co-developed the drug with AnHeart and led its approval and launch in China under the brand name Dovbleron®. In Japan, rights to Taletrectinib were licensed to Nippon Kayaku, a Japanese pharmaceutical company, for clinical development and commercialization.
How Does Taletrectinib work?
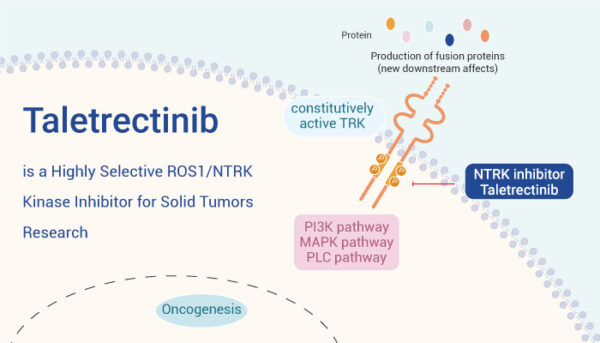
Taletrectinib is a next-generation tyrosine kinase inhibitor (TKI) that blocks the activity of abnormal ROS1 and NTRK fusion proteins—genetic changes that drive the growth of certain cancers. By targeting these fusions, Taletrectinib disrupts cancer cell signaling pathways, slowing or stopping tumor growth.
What sets Taletrectinib apart is its ability to overcome common resistance mutations, especially ROS1 G2032R, which often limits the effectiveness of older drugs like crizotinib. It also shows strong activity in the brain, helping treat tumors that have spread to the central nervous system. Unlike some other TRK inhibitors, Taletrectinib is more selective and may cause fewer neurological side effects, such as dizziness or balance issues.
Source: cancer research network
What Cancers Is Taletrectinib Approved to Treat?
Taletrectinib is approved for the treatment of adults with locally advanced or metastatic ROS1-positive non–small cell lung cancer (NSCLC). This approval applies to patients both with and without prior ROS1-targeted therapy.
The U.S. Food and Drug Administration (FDA) granted full approval to Taletrectinib on June 11, 2025, following a priority review process. In China, the drug is sold under the brand name Dovbleron and was approved by the National Medical Products Administration (NMPA) in December 2024. It became available to patients in early 2025. Meanwhile, in Japan, regulatory filings are underway, but marketing approval has not yet been granted.
At this time, Taletrectinib is approved only for ROS1-positive NSCLC, though additional studies are investigating its potential in other cancers driven by ROS1 or NTRK gene fusions.
What research is behind the approval?
The FDA approval of Taletrectinib for ROS1-positive non–small cell lung cancer (NSCLC) is based on results from two phase II clinical trials: TRUST-I (conducted in China) and TRUST-II (global). These open-label, single-arm studies evaluated Taletrectinib 600 mg once daily in 273 patients with advanced ROS1-positive NSCLC—160 treatment-naïve and 113 previously treated with ROS1-targeted therapies. Both trials assessed the drug’s systemic and intracranial efficacy, with the primary endpoint being confirmed objective response rate (cORR), and secondary endpoints including duration of response (DOR), progression-free survival (PFS), and safety. Results were presented at the 2025 ASCO Annual Meeting.
In treatment-naïve patients, Taletrectinib achieved a cORR of 88.8%, with a median DOR of 44.2 months and PFS of 45.6 months. Intracranial responses were also strong, with a 76.5% response rate. Among pretreated patients, the cORR was 55.8%, with median DOR of 16.6 months and PFS of 9.7 months. Intracranial cORR reached 65.6%.
Subgroup analyses showed consistent efficacy across race (Asian vs non-Asian), region, and chemotherapy history. Taletrectinib’s safety profile was manageable, with grade ≥3 adverse events seen in just over half of patients. Common side effects included elevated liver enzymes, nausea, diarrhea, and fatigue. Treatment discontinuation due to side effects was low (~6–7%). These data demonstrate that Taletrectinib delivers durable systemic and brain responses in both TKI-naïve and pretreated patients and support its role as a new targeted option for ROS1-positive NSCLC.

You can read more information about FDA approval of Taletrectinib for ROS1 positive Non Small Cell Lung Cancer on OncoDaily.
Taletrectinib side effects and its management
Like many targeted therapies, Ibtrozi can cause a range of side effects. Some are common and typically mild, while others are less frequent but potentially serious. Understanding these effects and how they’re managed is vital for safe and effective treatment.
Common Side Effects
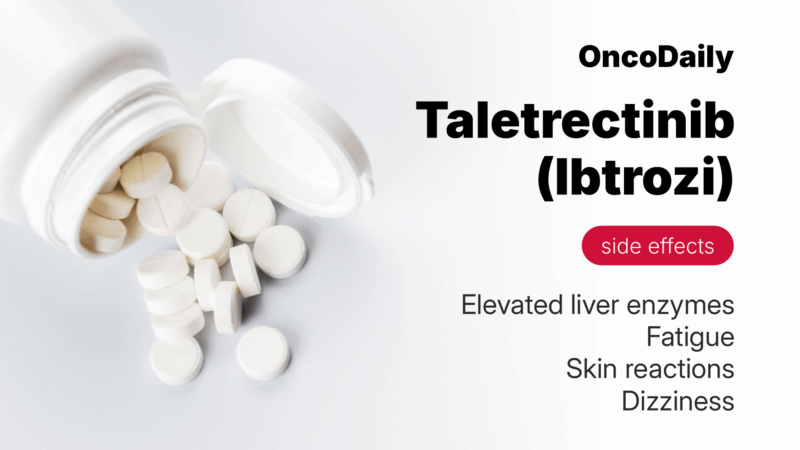
The most common side effects of Ibtrozi include gastrointestinal symptoms, fatigue, skin reactions, dizziness, and elevated liver enzymes. Diarrhea affects over half of patients, usually starting in the first weeks, and can be managed with hydration, diet changes, and anti-diarrheal medicines like loperamide. Nausea and vomiting are also frequent but typically controllable with anti-nausea drugs and taking the medication on an empty stomach.
Fatigue occurs in about 20% of patients and may range from mild tiredness to significant exhaustion. Gentle exercise, rest, and nutrition often help. Dizziness and light-headedness are usually mild and temporary, but patients should avoid driving if affected.
Skin rash, especially on sun-exposed areas, may develop and can be eased by using sunscreen and moisturizers. Elevated liver enzymes (ALT and AST) are common and usually mild, detected through routine blood tests. Other less frequent effects include appetite changes, dry mouth, and constipation, generally manageable with diet adjustments.
Less Common Side Effects
Less common but serious side effects include QT prolongation, requiring ECG monitoring and caution with other QT-prolonging drugs. Interstitial lung disease (ILD) or pneumonitis is rare but potentially severe, and new respiratory symptoms should be reported immediately.
Hyperuricemia and muscle-related issues like elevated CPK occur less often and are managed with monitoring and supportive care. Bone fractures have been reported, especially in those with osteoporosis risk, warranting bone health monitoring. Photosensitivity can cause sunburn or skin irritation; daily sunscreen use and protective clothing are advised.
Managing Side Effects
Managing side effects involves regular check-ups including blood tests and ECGs. Gastrointestinal symptoms can often be controlled with supportive medications. Elevated liver enzymes may require treatment pauses or dose reductions. Any new breathing difficulties or chest symptoms should be reported immediately for evaluation of lung toxicity. Patients should protect their skin from the sun to reduce rash and photosensitivity risks. Early communication with the healthcare team is essential to address side effects promptly and keep treatment on track.
What is the Recommended Dosage of Taletrectinib?
Taletrectinib is available as 200 mg capsules and is approved for treating locally advanced or metastatic ROS1-positive non–small cell lung cancer (NSCLC). The usual starting dose is 600 mg taken orally once daily, continued until disease progression or unacceptable side effects occur. If side effects arise, the dose may be reduced stepwise—from 600 mg to 400 mg daily, and then to 200 mg daily if needed. Treatment should be permanently stopped if patients cannot tolerate the lowest dose.
Specific side effects, such as interstitial lung disease (ILD), severe QT prolongation (a heart rhythm issue), elevated liver enzymes, or other significant toxicities, require temporarily holding Taletrectinib. Depending on recovery and severity, the drug may be resumed at the same or a reduced dose, or permanently discontinued if toxicity persists or recurs. Before starting treatment, doctors evaluate liver function, heart rhythm (with ECG), electrolytes, and uric acid levels to ensure safety. Taletrectinib is prescribed only to patients whose tumors have ROS1 rearrangements, though no FDA-approved test currently exists for detecting these alterations.
Learn more about Non-Small Cell Lung Cancer: Causes, Symptoms, Diagnosis, Treatment Options, and Latest 2025 Advances in Targeted and Immunotherapy on OncoDaily.
How is Taletrectinib administration?
Ibtrozi capsules should be taken orally on an empty stomach, avoiding food for at least two hours before and after the dose. Take the medication at the same time each day and swallow capsules whole—do not open, chew, or crush them. Avoid grapefruit and grapefruit-containing products during treatment. If using antacids, take them at least two hours apart from Taletrectinib. If a dose is missed or vomited, skip it and take the next dose as scheduled. Store the capsules at room temperature, ideally between 20–25ºC.
How is Taletrectinib Metabolized and Eliminated from the body?
Taletrectinib is mainly broken down by CYP3A enzymes, along with non-CYP450 pathways such as sulfation and acetylation. It is also partially metabolized by CYP2C8 and CYP2C9. The drug has a long half-life of approximately 66 hours, meaning it remains in the body for several days. Most of the drug is eliminated in the feces (75%), with 15% excreted unchanged, while about 11% is cleared through the urine, with 3% unchanged.
What to Avoid During Taletrectinib Treatment?
While taking Ibtrozi, patients should avoid eating or drinking grapefruit and grapefruit-containing products, as these can interfere with how the drug works. It’s also important to avoid taking antacids too close to the medication—leave at least two hours between them. Patients should be cautious with other medications that affect heart rhythm or liver function and always inform their healthcare provider about all drugs and supplements they are using. Lastly, because Taletrectinib can cause dizziness, patients should avoid activities requiring alertness, such as driving, if they feel light-headed.
Taletrectinib effectiveness over time
Ibtrozi has demonstrated strong and lasting effectiveness in treating ROS1-positive non–small cell lung cancer. Clinical trials show that patients who have not previously received ROS1-targeted therapies experience high response rates, with many enjoying prolonged disease control lasting several years. Even patients previously treated with other ROS1 inhibitors benefit, with meaningful responses and delayed progression.
The drug also works well against brain metastases, maintaining activity in the central nervous system over time. Regular monitoring helps ensure continued benefit while managing side effects. Overall, Ibtrozi offers durable control of ROS1-positive NSCLC, making it a valuable option for long-term treatment.
Ongoing trials with Taletrectinib
A Phase II clinical trial (NCT06214793) is investigating the safety and effectiveness of Taletrectinib in patients with metastatic invasive lobular breast cancer (ILC) that carries a mutation in the CDH1 gene. This mutation, common in about 75% of ILC cases, affects a protein called E-cadherin, which helps cells stick together. Preclinical studies suggest that CDH1 mutations increase ROS1 activity, making ROS1 inhibitors like Taletrectinib potentially effective against these tumors. The trial focuses on patients who have already received prior treatments, aiming to see how well Taletrectinib works in this specific breast cancer subtype.
The ongoing real-world study (NCT07008287), sponsored by Innovent Biologics, is evaluating the effectiveness and safety of Taletrectinib in patients with advanced ROS1-positive non-small cell lung cancer (NSCLC) who also have brain metastases. The study is observational and non-interventional, meaning Taletrectinib is given as part of routine care based on the physician’s decision. Researchers are collecting data on treatment patterns, clinical outcomes, and side effects during standard follow-up visits. The goal is to better understand how Taletrectinib performs in everyday clinical settings, especially in patients with brain involvement.
Written by Mariam Khachatryan, MD
FAQ
What is Taletrectinib used for?
Ibtrozi is used to treat adults with locally advanced or metastatic non–small cell lung cancer (NSCLC) whose tumors carry ROS1 gene fusions.
How does Taletrectinib differ from older ROS1 inhibitors?
Unlike earlier drugs like crizotinib, Ibtrozi is designed to overcome resistance mutations, such as ROS1 G2032R. It also shows stronger activity in the brain and may cause fewer neurological side effects due to its selective targeting.
Can Taletrectinib be used after other ROS1-targeted therapies?
Yes. Taletrectinib has shown clinical benefit even in patients who were previously treated with other ROS1 inhibitors, making it a promising option for both first-line and later-line therapy.
How long does Taletrectinib stay in the body?
Taletrectinib has a long half-life of about 66 hours, allowing for once-daily dosing. Its effects persist over several days, helping maintain consistent cancer control with regular use.
What should I do if I miss a dose of Taletrectinib?
If a dose is missed or vomited, patients should not take an extra pill. Instead, they should skip that dose and take the next one at the usual time the following day.
How is Taletrectinib metabolized in the body?
Taletrectinib is primarily broken down by liver enzymes such as CYP3A, and to a lesser extent by CYP2C8 and CYP2C9.
What are the most common side effects of Ibtrozi?
Common side effects include diarrhea, nausea, fatigue, dizziness, and elevated liver enzymes. Most are manageable with supportive care, but regular checkups are important to monitor for more serious issues like lung toxicity or heart rhythm changes.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
