Cisplatin, discovered in the 1960s and approved for clinical use in the 1970s, remains one of the most widely utilized platinum-based chemotherapeutic agents. Its unique DNA-damaging mechanism has made it a cornerstone in the treatment of various solid tumors, including testicular, ovarian, bladder, cervical, lung, and head and neck cancers. Despite the development of newer agents, cisplatin continues to be included in standard-of-care protocols recommended by major oncology guidelines such as NCCN and ESMO. Its proven efficacy, particularly in curable malignancies like testicular cancer, underscores its ongoing clinical importance. However, its use is limited by significant toxicities and the emergence of resistance, necessitating a deeper understanding of its pharmacologic profile within the evolving framework of precision oncology.
This article will explore the pharmacodynamics, clinical applications, resistance mechanisms, toxicity profile, and current strategies to enhance the efficacy and safety of cisplatin.
Mechanism of Action of Cisplatin: DNA Damage and Cellular Response
Cisplatin exerts its cytotoxic effect primarily through the formation of covalent DNA adducts. After entering the cell, cisplatin undergoes aquation, a process in which its chloride ligands are replaced by water molecules in the low-chloride intracellular environment. This transformation activates the molecule, allowing it to bind to nucleophilic sites on DNA, particularly the N7 position of guanine bases. The result is the formation of intrastrand and interstrand DNA crosslinks, predominantly between adjacent purines, which distort the DNA helix and obstruct essential processes such as replication and transcription.
The accumulation of such DNA lesions activates the DNA damage response (DDR), leading to cell cycle arrest, particularly at the G2/M checkpoint, and ultimately apoptosis. This effect is especially pronounced in rapidly dividing cells, which rely heavily on uninterrupted DNA synthesis. In addition to DNA-directed mechanisms, cisplatin induces cytotoxicity through secondary pathways, including the generation of reactive oxygen species (ROS), oxidative stress, and mitochondrial dysfunction—processes that further promote apoptotic cell death.
Cisplatin sensitivity is strongly influenced by the cellular context, particularly the functionality of the tumor suppressor protein p53, which mediates apoptosis in response to DNA damage. Additionally, the mismatch repair (MMR) system plays a key role in recognizing cisplatin-DNA adducts; defects in this pathway can lead to tolerance of DNA lesions and contribute to chemoresistance.
Pharmacokinetics and Pharmacodynamics of Cisplatin
Cisplatin exhibits distinct pharmacokinetic characteristics that influence both its therapeutic efficacy and toxicity profile. Due to its poor oral bioavailability, cisplatin is administered exclusively via intravenous infusion. Upon systemic administration, it is rapidly distributed throughout the body and demonstrates a strong affinity for plasma proteins, with more than 90% of circulating cisplatin becoming protein-bound within a few hours. This high degree of binding limits the amount of free, active drug available in circulation but prolongs its retention in the body.
The distribution of cisplatin is non-uniform and shows preferential accumulation in specific tissues, notably the kidneys, liver, and inner ear. This selective tissue uptake underlies the organ-specific toxicities commonly associated with cisplatin therapy, such as nephrotoxicity, hepatotoxicity, and ototoxicity. The renal cortex, in particular, demonstrates high cisplatin concentration, which correlates with the drug’s dose-limiting renal toxicity.
Unlike many chemotherapeutic agents, cisplatin undergoes non-enzymatic biotransformation. After aquation, the active hydrated species react with cellular nucleophiles, including DNA and thiol-containing molecules like glutathione. This interaction results in the inactivation of cisplatin and contributes to resistance and detoxification. The primary route of elimination is via the kidneys, with more than 90% of the drug being excreted in the urine, mostly in a protein-bound or metabolized form. As a result, renal function significantly affects cisplatin clearance, and dosing must be carefully adjusted in patients with renal impairment to prevent accumulation and toxicity.
Cisplatin demonstrates a biphasic plasma half-life: an initial distribution phase with a short half-life of 20–30 minutes, followed by a prolonged elimination phase with a terminal half-life of approximately 20–30 hours due to strong tissue binding. The free (unbound) form of cisplatin is pharmacologically active and primarily responsible for cytotoxic effects, while the protein-bound form is considered largely inactive but contributes to prolonged tissue exposure and delayed toxicities.
Clinical Applications of Cisplatin in Cancer Therapy
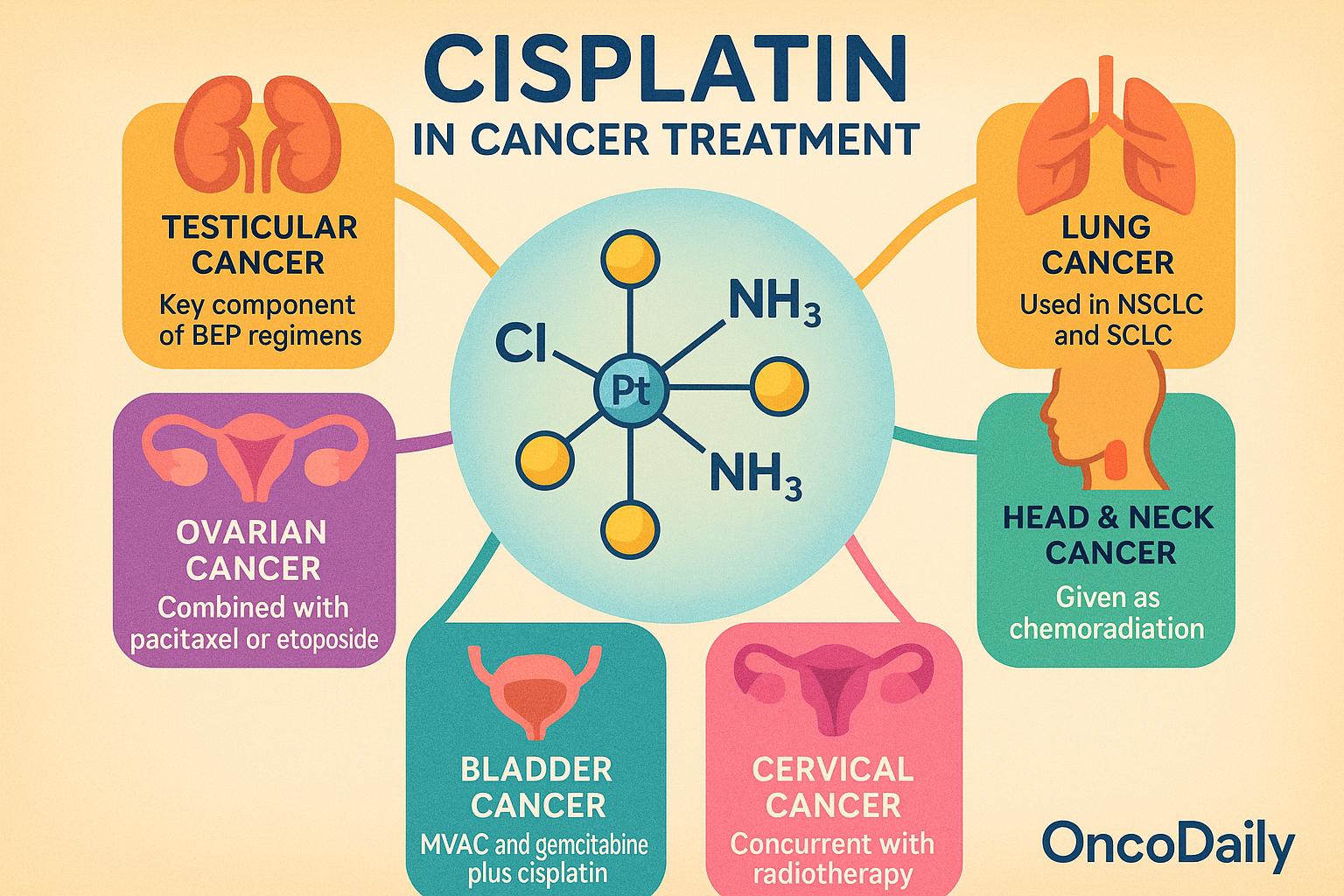
Cisplatin remains a cornerstone chemotherapeutic agent in the treatment of multiple solid tumors, owing to its broad-spectrum antineoplastic activity and synergistic effects when combined with other cytotoxic or radiation-based therapies. It is used both as monotherapy and more commonly as part of combination regimens across a range of malignancies.
In testicular cancer, cisplatin is the key component of curative regimens such as BEP (bleomycin, etoposide, and cisplatin). Its introduction has transformed metastatic testicular cancer from a fatal disease to one with cure rates exceeding 90%, particularly in patients with favorable-risk germ cell tumors. In ovarian cancer, cisplatin is used in both first-line and salvage settings, often combined with paclitaxel or etoposide. It contributes significantly to disease control, particularly in platinum-sensitive recurrences.
Lung cancer therapy frequently incorporates cisplatin in both non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). In NSCLC, cisplatin is used in combination with agents such as pemetrexed, gemcitabine, or vinorelbine, especially in younger or fitter patients. In SCLC, it is commonly paired with etoposide and used in both limited and extensive-stage disease. In head and neck squamous cell carcinoma, cisplatin is the preferred agent for concurrent chemoradiation in definitive or adjuvant settings. It enhances the radiosensitivity of tumor cells, leading to improved locoregional control and survival.
In bladder cancer, cisplatin-based combinations such as MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) or gemcitabine plus cisplatin are the standard of care in neoadjuvant and metastatic settings. Cisplatin has demonstrated superior survival outcomes compared to non-platinum alternatives, particularly in cisplatin-eligible patients. For cervical cancer, cisplatin is administered concurrently with radiotherapy as the standard chemoradiation approach for locally advanced disease. Its radiosensitizing properties improve both progression-free and overall survival.
Cisplatin is utilized across therapeutic contexts—neoadjuvant, adjuvant, and palliative—depending on tumor type and disease stage. It is especially preferred over carboplatin in clinical settings where achieving higher response rates or complete remission is critical, such as in germ cell tumors and certain locally advanced cancers. However, when contraindications such as renal impairment, hearing loss, or poor performance status are present, carboplatin may be substituted, albeit with generally reduced efficacy.
Mechanisms of Cisplatin Resistance: Cellular Adaptations and Therapeutic Challenges
Resistance to cisplatin, whether intrinsic (present before treatment) or acquired (developing during therapy), remains a major obstacle to its long-term clinical effectiveness. Tumor cells employ a multifaceted array of mechanisms to evade cisplatin-induced cytotoxicity, which involve alterations in drug transport, detoxification, DNA repair, and cell death pathways.
One of the earliest adaptations seen in resistant cells is a reduction in intracellular cisplatin accumulation. This can occur through decreased uptake via the copper transporter CTR1, which normally facilitates cisplatin entry into the cell, or through increased efflux mediated by ATP-binding cassette (ABC) transporters such as MRP2 (multidrug resistance-associated protein 2). These alterations effectively reduce the intracellular concentration of active drug, limiting DNA damage.
In parallel, enhanced detoxification mechanisms contribute to resistance. Tumor cells often upregulate the synthesis of glutathione (GSH) and metallothioneins, which are thiol-rich molecules capable of binding and neutralizing cisplatin before it can reach nuclear DNA. This process results in the inactivation of cisplatin through conjugation and subsequent export out of the cell.
Another key contributor is enhanced DNA repair capacity, particularly through the nucleotide excision repair (NER)pathway, which removes cisplatin-induced intrastrand adducts. Overexpression of the NER enzyme ERCC1-XPFcomplex is strongly associated with resistance and poor treatment outcomes in several cancers. Additionally, increased activity of homologous recombination (HR) repair mechanisms, especially involving BRCA1/2 and RAD51, can repair the more cytotoxic interstrand crosslinks and double-strand breaks, further reducing cisplatin efficacy.
Beyond DNA repair, alterations in cell death signaling are crucial. Many resistant tumors exhibit upregulation of anti-apoptotic proteins such as Bcl-2, Bcl-xL, and inhibitors of apoptosis proteins (IAPs), which suppress cisplatin-triggered apoptotic pathways. Furthermore, autophagy, a cellular stress response mechanism, is increasingly recognized as a survival strategy in cisplatin-treated cells. While initially thought to be protective, dysregulated autophagy can facilitate resistance by degrading damaged organelles and recycling nutrients.
These resistance mechanisms are not mutually exclusive and often occur in combination, contributing to treatment failure and disease progression. Understanding these pathways has become a focus of translational and clinical research, with efforts aimed at identifying predictive biomarkers of response and developing co-targeting strategies. Therapeutic approaches under investigation include inhibitors of efflux transporters, glutathione synthesis blockers, NER or HR pathway inhibitors, and pro-apoptotic agents, which may help to overcome resistance and restore cisplatin sensitivity in refractory tumors.
Toxicity Profile of Cisplatin: Balancing Efficacy and Safety
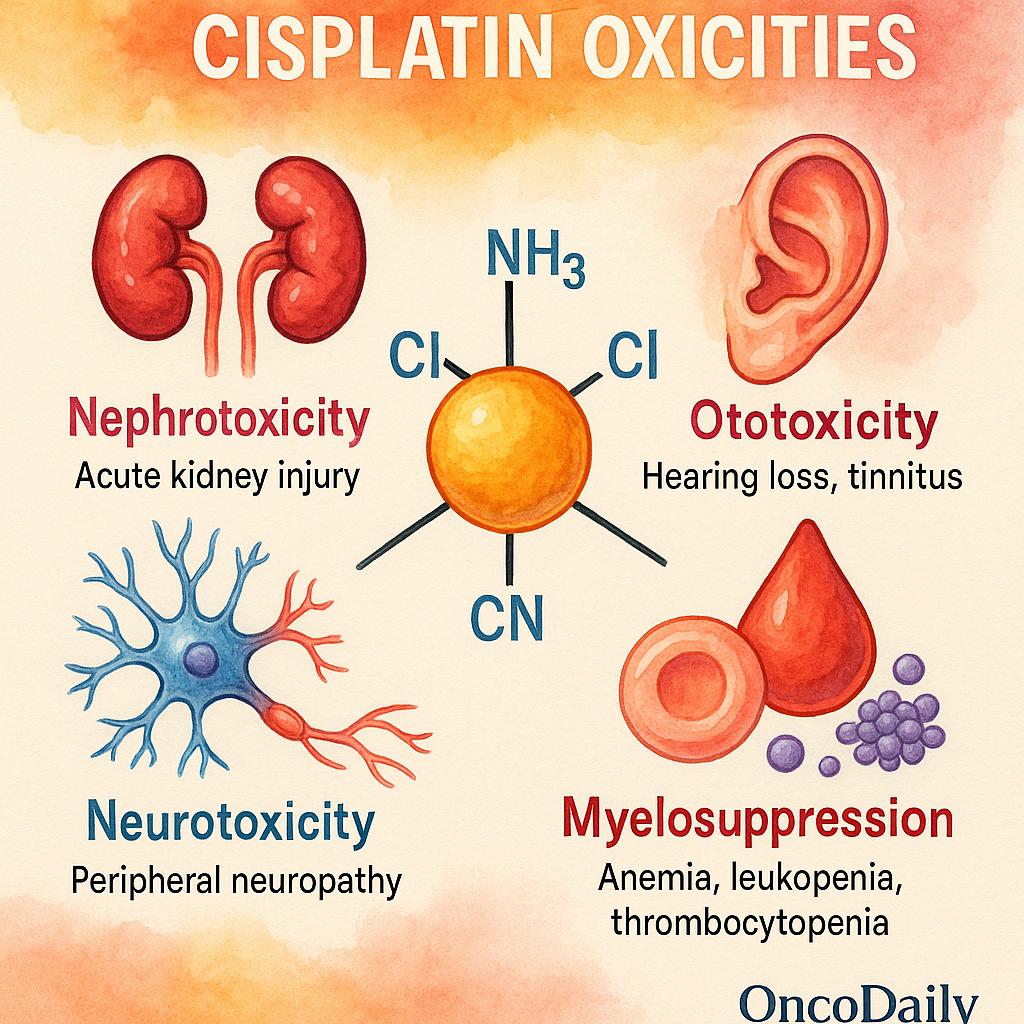
Cisplatin’s clinical utility is limited by a range of dose-limiting toxicities, which are often cumulative and can significantly impact both quality of life and treatment adherence. These toxicities necessitate careful monitoring, supportive care measures, and, in some cases, dose modifications to optimize outcomes.
The most significant and common toxicity is nephrotoxicity, primarily due to proximal tubular damage in the kidneys. Cisplatin accumulates in renal tubular epithelial cells, leading to oxidative stress, inflammation, and apoptosis. If unmitigated, this can result in acute kidney injury or chronic renal dysfunction. To reduce this risk, prehydration protocols with isotonic saline, often supplemented with mannitol for forced diuresis, are routinely employed. Additionally, nephroprotective agents like amifostine may be considered in selected cases, although their use is balanced against their own side effects and cost.
Ototoxicity is another serious and often irreversible adverse effect, presenting as bilateral high-frequency hearing loss and tinnitus. This toxicity is particularly concerning in pediatric patients, where it can impair language development and learning. The mechanism is thought to involve damage to the cochlear hair cells and stria vascularis. There are currently no FDA-approved otoprotectants, though investigational agents such as sodium thiosulfate have shown promise in clinical trials.
Neurotoxicity, in the form of peripheral neuropathy, is frequently seen with cumulative dosing and often manifests as sensory deficits, paresthesia, or proprioceptive loss. Recovery is slow and incomplete in many patients, and severe cases can become dose-limiting. Early recognition and dose reduction are key to minimizing long-term disability.
While myelosuppression is generally less pronounced with cisplatin compared to other chemotherapeutics, anemia, leukopenia, and thrombocytopenia can still occur, particularly in combination regimens or in patients with prior bone marrow compromise. Monitoring of complete blood counts is standard, and supportive treatments such as growth factors may be used when appropriate.
Cisplatin is also highly emetogenic, leading to intense nausea and vomiting if not properly managed. Modern antiemetic protocols typically include 5-HT3 receptor antagonists (e.g., ondansetron), corticosteroids (e.g., dexamethasone), and neurokinin-1 (NK1) receptor antagonists (e.g., aprepitant) to provide effective prophylaxis and control.
To mitigate these toxicities, frequent monitoring of renal function, audiometry (especially in younger patients), neurologic assessments, and hematologic parameters is essential. Dose adjustments based on creatinine clearance, performance status, and prior toxicity are commonly employed to individualize treatment. The clinical challenge lies in achieving a therapeutic balance—maximizing the cytotoxic benefit of cisplatin while minimizing irreversible organ damage that can compromise long-term health.
 Strategies to Enhance Cisplatin Efficacy and Reduce Toxicity
Strategies to Enhance Cisplatin Efficacy and Reduce Toxicity
Ongoing efforts in cancer research are increasingly focused on improving the therapeutic index of cisplatin—enhancing its antitumor activity while minimizing associated toxicities. These strategies span drug delivery innovations, rational combination therapies, biomarker integration, and personalized medicine approaches.
One promising avenue is the development of nanoparticle-based and liposomal formulations of cisplatin designed to improve drug delivery and reduce systemic toxicity. By encapsulating cisplatin within nanocarriers, these systems aim to increase selective accumulation in tumor tissue via the enhanced permeability and retention (EPR) effect while minimizing off-target distribution to organs such as the kidneys and inner ear. Preclinical models and early-phase trials have demonstrated that these formulations can achieve comparable or improved efficacy with reduced nephrotoxicity and neurotoxicity.
Cisplatin is also being increasingly integrated into combination regimens with targeted therapies (such as EGFR or VEGF inhibitors) and immune checkpoint inhibitors (such as PD-1/PD-L1 antibodies). These combinations are designed to exploit cisplatin’s immunogenic and pro-apoptotic effects while simultaneously blocking pathways that mediate resistance or immune escape. In cancers such as non-small cell lung cancer and bladder cancer, cisplatin-based chemoimmunotherapy regimens are under active clinical investigation and have shown early success in improving response rates and survival.
A major advance in cisplatin optimization is the use of predictive biomarkers for patient selection. The DNA repair enzyme ERCC1 (excision repair cross-complementation group 1), which mediates nucleotide excision repair, has been extensively studied as a negative predictor of cisplatin efficacy. High ERCC1 expression is associated with enhanced DNA repair capacity and resistance to cisplatin, prompting trials that stratify patients by ERCC1 status to guide treatment decisions.
Pharmacogenomics is also gaining traction in cisplatin-based therapy. Polymorphisms in genes involved in drug transport (e.g., SLC22A2), detoxification (e.g., GSTP1), and DNA repair (e.g., XRCC1) have been linked to interindividual variability in both toxicity and response. Tailoring cisplatin dosing or selection based on a patient’s genomic profile may allow for more precise therapy that maximizes benefit while minimizing harm.
Beyond molecular personalization, dose-intensity and schedule modifications are being refined to improve tolerability without compromising efficacy. Fractionated dosing schedules, split-dose regimens, and weekly low-dose cisplatin have been explored to reduce cumulative toxicity while maintaining antitumor activity. In parallel, clinical trials are testing synergistic combinations with radiosensitizers, metabolic inhibitors, and novel cytotoxics to broaden the therapeutic scope of cisplatin.
Immunomodulatory Effects of Cisplatin and Its Synergy with Immunotherapy
Traditionally viewed as a DNA-damaging cytotoxic agent, cisplatin is now increasingly recognized for its immunomodulatory properties, which have important implications for combination strategies with immune-based therapies. Emerging preclinical and clinical evidence suggests that cisplatin not only kills cancer cells directly but also modulates the tumor immune microenvironment (TME) in ways that can enhance antitumor immunity.
One of the key immunologic actions of cisplatin is the induction of immunogenic cell death (ICD). Unlike non-immunogenic apoptosis, ICD leads to the release of damage-associated molecular patterns (DAMPs), including calreticulin, ATP, and HMGB1, which activate dendritic cells and promote antigen presentation. This, in turn, enhances cytotoxic T cell priming and adaptive immune responses against tumor-associated antigens.
Cisplatin also facilitates upregulation of MHC class I molecules and tumor antigens on cancer cells, making them more visible to cytotoxic T lymphocytes. In addition, it can increase the infiltration of tumor-infiltrating lymphocytes (TILs), including CD8+ T cells and NK cells, while potentially depleting immunosuppressive populations such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs).
These immunogenic effects form the biological rationale for combining cisplatin with immune checkpoint inhibitors (ICIs), such as anti-PD-1 or anti-PD-L1 antibodies. Cisplatin-induced immune priming may enhance the responsiveness of tumors to ICIs by converting a “cold” (non-immunogenic) tumor into a “hot” (immunologically active) one, thereby overcoming one of the major barriers to immunotherapy efficacy.
Clinical trials in non-small cell lung cancer (NSCLC) and bladder cancer have provided some of the most compelling examples of cisplatin’s synergy with immunotherapy. In the IMpower133 trial for extensive-stage small cell lung cancer, platinum-based chemotherapy (carboplatin or cisplatin) combined with atezolizumab (anti-PD-L1) significantly improved overall survival compared to chemotherapy alone. Similarly, in bladder cancer, the combination of cisplatin-based chemotherapy (e.g., gemcitabine + cisplatin) with immune checkpoint inhibitors is being actively explored in both neoadjuvant and metastatic settings, with early data showing promising improvements in pathologic complete response and durable disease control.
These findings reinforce the dual role of cisplatin as both a cytotoxic and immunomodulatory agent, and they support its integration into chemoimmunotherapy regimens as a strategy to enhance the efficacy of cancer immunotherapy. Ongoing trials are expected to clarify the optimal sequencing, dosing, and patient selection criteria to maximize the immunogenic potential of cisplatin in combination with ICIs.
Platinum Analogs: Alternatives to Cisplatin and Their Clinical Role
To address the limitations of cisplatin—particularly its nephrotoxicity, ototoxicity, and acquired resistance—a range of platinum analogs have been developed. These agents aim to retain the antitumor activity of cisplatin while offering improved safety profiles or activity in cisplatin-resistant tumors.
Carboplatin is the most commonly used alternative. It has a more favorable toxicity profile, particularly with respect to renal and auditory toxicity, making it suitable for cisplatin-ineligible patients, such as those with pre-existing kidney dysfunction or poor performance status. However, carboplatin is less potent, and its use is generally associated with lower response rates in tumors like testicular cancer and head and neck squamous cell carcinoma, where cisplatin remains superior.
Oxaliplatin, another analog, is structurally distinct and used primarily in colorectal and gastric cancers, often in regimens such as FOLFOX or XELOX. While it avoids nephrotoxicity, its main limitation is peripheral sensory neuropathy, which can be dose-limiting and persistent. Oxaliplatin has shown minimal cross-resistance with cisplatin and carboplatin, which has expanded its use in tumors not typically sensitive to cisplatin.
Several investigational agents have been evaluated for further improvement:
-
Picoplatin was designed to overcome resistance mediated by glutathione conjugation, but clinical trials have not demonstrated a clear advantage.
-
Nedaplatin, used mainly in Asia, offers reduced nephrotoxicity and hematologic toxicity compared to cisplatin and has shown promise in head and neck and cervical cancers.
-
Satraplatin, an orally bioavailable platinum compound, showed some activity in prostate cancer but failed to gain widespread clinical use due to limited efficacy and regulatory hurdles.
Despite these alternatives, cisplatin remains the gold standard in several tumor types—particularly testicular, bladder, head and neck, and cervical cancers—due to its higher response rates and curative potential. In these settings, substitutes are typically reserved for patients who cannot tolerate cisplatin rather than as equal alternatives.
Future Directions in Cisplatin Research and Clinical Optimization
Current research efforts surrounding cisplatin are focused on improving its efficacy, overcoming resistance, and tailoring its use through personalized oncology strategies. One major area of interest is the development of cisplatin-based combination regimens with novel agents, including PARP inhibitors, DNA damage response modulators, epigenetic drugs, and immune checkpoint inhibitors. These combinations aim to enhance cisplatin’s cytotoxic effects, sensitize resistant tumors, and extend its clinical utility to new cancer types.
Understanding cisplatin resistance at a deeper level is also a research priority. Investigators are employing single-cell transcriptomics, proteomics, and multi-omics approaches to map the heterogeneity of resistance mechanisms across tumor subpopulations. These technologies are revealing dynamic adaptations in DNA repair pathways, metabolic reprogramming, and immune evasion that contribute to resistance, offering potential new targets for intervention.
To better model cisplatin response and resistance, researchers are using patient-derived organoids and patient-derived xenograft (PDX) models, which more accurately replicate the tumor microenvironment and genetic diversity of human cancers. These systems allow for functional testing of drug sensitivity and serve as platforms for preclinical evaluation of combination therapies.
In parallel, real-world data analyses are being conducted to complement findings from clinical trials. These studies focus on long-term outcomes, including survival, quality of life, and cumulative toxicity, and help identify clinical predictors of response and tolerability across diverse patient populations. Real-world evidence is particularly valuable in guiding cisplatin use among elderly, frail, or comorbid patients who are often underrepresented in trials.
A consistent theme across these research domains is the urgent need for robust predictive biomarkers. Markers such as ERCC1, BRCA1/2, TP53 mutations, and others are under investigation to identify patients most likely to benefit from cisplatin and those at high risk of resistance or severe toxicity. These efforts align with the broader goal of developing more personalized treatment algorithms, where cisplatin use is informed by molecular profiling and functional assays rather than one-size-fits-all protocols.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD


