Hormonal therapy represents a fundamental strategy in the treatment of hormone-dependent malignancies, particularly breast, prostate, and endometrial cancers. By targeting the hormonal signaling pathways that drive tumor growth and survival, this therapeutic approach has transformed outcomes across both early-stage and metastatic disease settings.
The concept of hormone responsiveness in cancer dates back over a century, with clinical observations that surgical removal of hormone-producing organs could induce tumor regression. These landmark findings catalyzed the development of pharmacologic interventions that either suppress hormone synthesis or disrupt receptor-mediated signaling, evolving into the diverse class of endocrine therapies used today. Unlike general hormone supplementation or deprivation, hormonal therapy in oncology involves precise modulation of endocrine axes to inhibit tumor-promoting mechanisms. Its clinical success has prompted deeper exploration into mechanisms of resistance, combination regimens, and biomarker-guided personalization.
This article provides a comprehensive overview of hormonal therapy in cancer treatment—detailing its biological foundations, mechanisms of action, clinical applications, resistance pathways, emerging agents, and future directions within precision oncology.
Biological Foundations of Hormone-Dependent Cancers
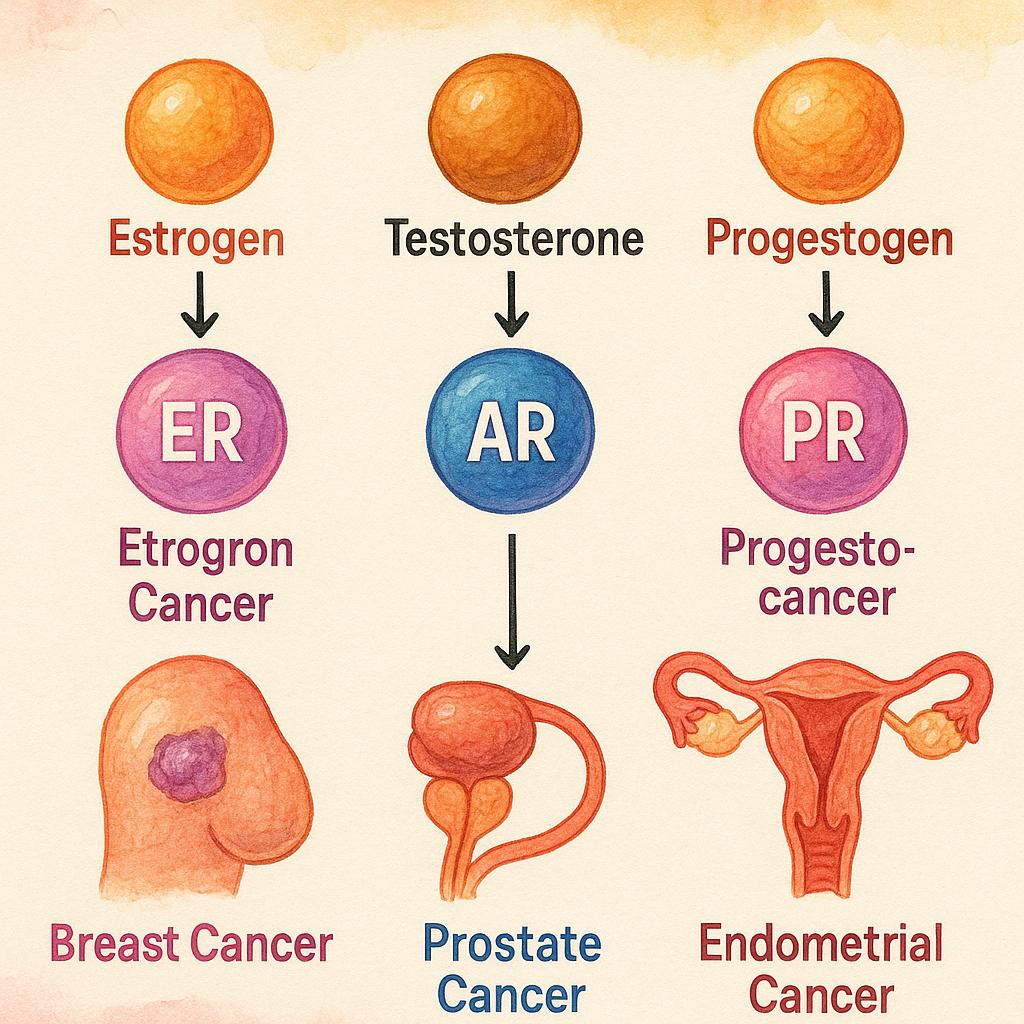
Endogenous hormones such as estrogens, androgens, and progestogens play a central role in regulating cellular proliferation, differentiation, and survival in various tissues. Under normal physiological conditions, these hormones bind to their respective nuclear receptors—such as estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR)—to modulate gene expression and maintain tissue homeostasis. However, in certain malignancies, these same pathways are hijacked to promote uncontrolled growth and resistance to apoptosis.
In estrogen receptor-positive (ER+) and/or progesterone receptor-positive (PR+) breast cancer, estrogen signaling serves as a primary driver of tumor proliferation. Upon binding to estrogen, ER undergoes conformational activation, translocates to the nucleus, and binds to estrogen response elements (EREs) within DNA. This promotes the transcription of genes involved in cell cycle progression, angiogenesis, and survival. As a result, ER+ breast cancers are highly dependent on estrogenic signaling for growth, making them susceptible to endocrine interventions.
Similarly, in prostate cancer, the androgen receptor (AR) plays a pivotal role. Testosterone and its more potent metabolite dihydrotestosterone (DHT) bind to AR, triggering receptor dimerization and nuclear translocation. Once in the nucleus, AR binds to androgen response elements (AREs) and regulates the transcription of genes critical for cell proliferation and survival. This androgen-driven transcriptional activity underlies the rationale for androgen deprivation therapy (ADT) in the management of prostate cancer.
Beyond breast and prostate cancers, rarer hormone-responsive tumors also demonstrate hormone dependency. For example, granulosa cell tumors of the ovary produce estrogen and are influenced by hormonal signaling, while endometrial cancers—especially those of the endometrioid subtype—often rely on unopposed estrogen stimulation in the absence of progesterone counter-regulation.
Mechanistic Classes of Hormonal Therapy in Oncology
Hormonal therapy in oncology functions through several distinct but interrelated mechanisms that target the hormone-driven pathways essential for tumor growth and progression. Rather than organizing treatment options by drug names, it is more scientifically relevant to classify them based on their mode of action, which is tailored according to tumor biology and the patient’s hormonal milieu.
One of the principal strategies is receptor antagonism or blockade, where therapeutic agents bind competitively to hormone receptors—such as the estrogen receptor (ER) in breast cancer or the androgen receptor (AR) in prostate cancer—thereby preventing endogenous hormones from activating these receptors. This inhibition disrupts the transcriptional activity that would otherwise drive proliferation, survival, and tumor progression. Receptor antagonists effectively silence hormone signaling at the nuclear level, especially in tumors that retain functional receptors.
Another core mechanism is hormone depletion, aimed at reducing the systemic levels of circulating hormones that tumors depend on. In premenopausal women with hormone receptor-positive breast cancer, ovarian suppression is achieved via gonadotropin-releasing hormone (GnRH) agonists or antagonists, which inhibit the hypothalamic-pituitary-gonadal axis and halt estrogen production. In men with prostate cancer, androgen deprivation is implemented using luteinizing hormone-releasing hormone (LHRH) analogs or surgical orchiectomy to lower testosterone and dihydrotestosterone levels. In postmenopausal women, where estrogen is primarily synthesized in peripheral tissues via aromatase, aromatase inhibitors are used to prevent this conversion, effectively reducing estrogen bioavailability.
A more refined approach involves selective receptor modulators and degraders, which influence receptor activity not only by blocking hormone binding but by inducing conformational changes that affect receptor stability and downstream signaling. Selective estrogen receptor modulators (SERMs) can function as antagonists or agonists depending on the tissue context, while selective estrogen receptor degraders (SERDs) promote ER degradation, leading to long-term inhibition of estrogenic signaling. Similarly, selective androgen receptor modulators (SARMs) are in development to modulate AR activity with potentially fewer side effects. These agents offer a sophisticated means to overcome receptor-mediated resistance, particularly in tumors with altered receptor dynamics or partial hormone independence.
A final and essential class of hormonal therapies includes enzyme inhibitors, which block the biosynthetic pathways responsible for hormone production. Aromatase inhibitors prevent the peripheral conversion of androgens into estrogens, particularly relevant in postmenopausal breast cancer. In prostate cancer, inhibitors of CYP17A1, such as abiraterone, interfere with the synthesis of androgens from cholesterol precursors, suppressing both adrenal and intratumoral androgen production.
Crucially, the selection of a hormonal therapy mechanism is not uniform across patients but is determined by the biological characteristics of the tumor—including receptor status, intrinsic resistance mechanisms, and expression of steroidogenic enzymes—as well as by the patient’s endocrine environment. For instance, a premenopausal woman with ER-positive breast cancer requires a different endocrine strategy than a postmenopausal patient, and a castration-resistant prostate tumor may necessitate enzyme inhibition in addition to receptor blockade. Understanding these mechanistic distinctions enables personalized treatment planning and sets the foundation for rational combinations with other therapeutic modalities.
Clinical Applications of Hormonal Therapy by Cancer Type
The clinical use of hormonal therapy is most established in cancers with well-characterized hormone receptor expression. Treatment strategies vary not only by tumor type but also by disease stage, menopausal or hormonal status, and risk stratification. Below is an overview of how hormonal therapy is currently applied in major hormone-driven cancers, with a focus on clinical indications, treatment goals, and supporting evidence.
In hormone receptor–positive breast cancer, endocrine therapy is a mainstay across early-stage, adjuvant, and metastatic settings. In early breast cancer, adjuvant hormonal therapy significantly reduces the risk of recurrence and improves survival. The choice of agent is guided by menopausal status: tamoxifen is typically used in premenopausal women, while aromatase inhibitors (AIs) are preferred in postmenopausal patients. For high-risk premenopausal patients, ovarian suppression with GnRH analogs is added to tamoxifen or an AI. In the metastatic setting, hormonal therapy remains first-line unless there is visceral crisis. Emerging agents such as selective estrogen receptor degraders (SERDs) are increasingly used in endocrine-resistant disease, particularly in patients with ESR1 mutations. Treatment duration in early-stage disease typically extends to 5–10 years, based on recurrence risk, with extended therapy reserved for those with high-risk features. Risk stratification tools, including genomic assays and clinical-pathological scoring systems, are frequently employed to guide these decisions.
In prostate cancer, hormonal therapy in the form of androgen deprivation therapy (ADT) remains the cornerstone of treatment. ADT is used across various stages, including localized high-risk disease, biochemical recurrence, and metastatic settings. In metastatic castration-sensitive prostate cancer, ADT is combined with novel agents such as androgen receptor inhibitors (e.g., enzalutamide, apalutamide) or androgen biosynthesis inhibitors (e.g., abiraterone), improving overall survival compared to ADT alone. In metastatic castration-resistant prostate cancer (mCRPC), further hormonal manipulation remains viable, with sequencing or combining second-line hormonal agents. Recent clinical trials support the use of triplet therapy—ADT, docetaxel, and a novel AR-targeted agent—in selected patients with high-volume disease. ADT is associated with significant long-term toxicity, so balancing oncologic benefit with quality-of-life considerations is critical, particularly in older patients or those with comorbidities.
In endometrial cancer, hormonal therapy is primarily reserved for low-grade, hormone receptor–positive tumors, particularly in early-stage disease or in patients seeking fertility preservation. Progestins such as medroxyprogesterone acetate or megestrol acetate are commonly used, often in combination with intrauterine devices delivering local hormone therapy. Hormonal therapy may also be used in advanced or recurrent endometrial cancer, especially when chemotherapy is contraindicated. Anti-estrogens or aromatase inhibitors have also shown activity in this setting. Although evidence is more limited compared to breast or prostate cancer, selected patients derive meaningful benefit with a relatively favorable toxicity profile.
In rarer hormone-responsive malignancies, such as ovarian granulosa cell tumors, hormonal therapy may be considered in recurrent or unresectable cases. These tumors are often estrogen-secreting and express hormone receptors, providing a rationale for anti-estrogen therapy, although high-level evidence is lacking and treatment remains individualized.
Mechanisms of Resistance to Hormonal Therapy and Adaptive Escape
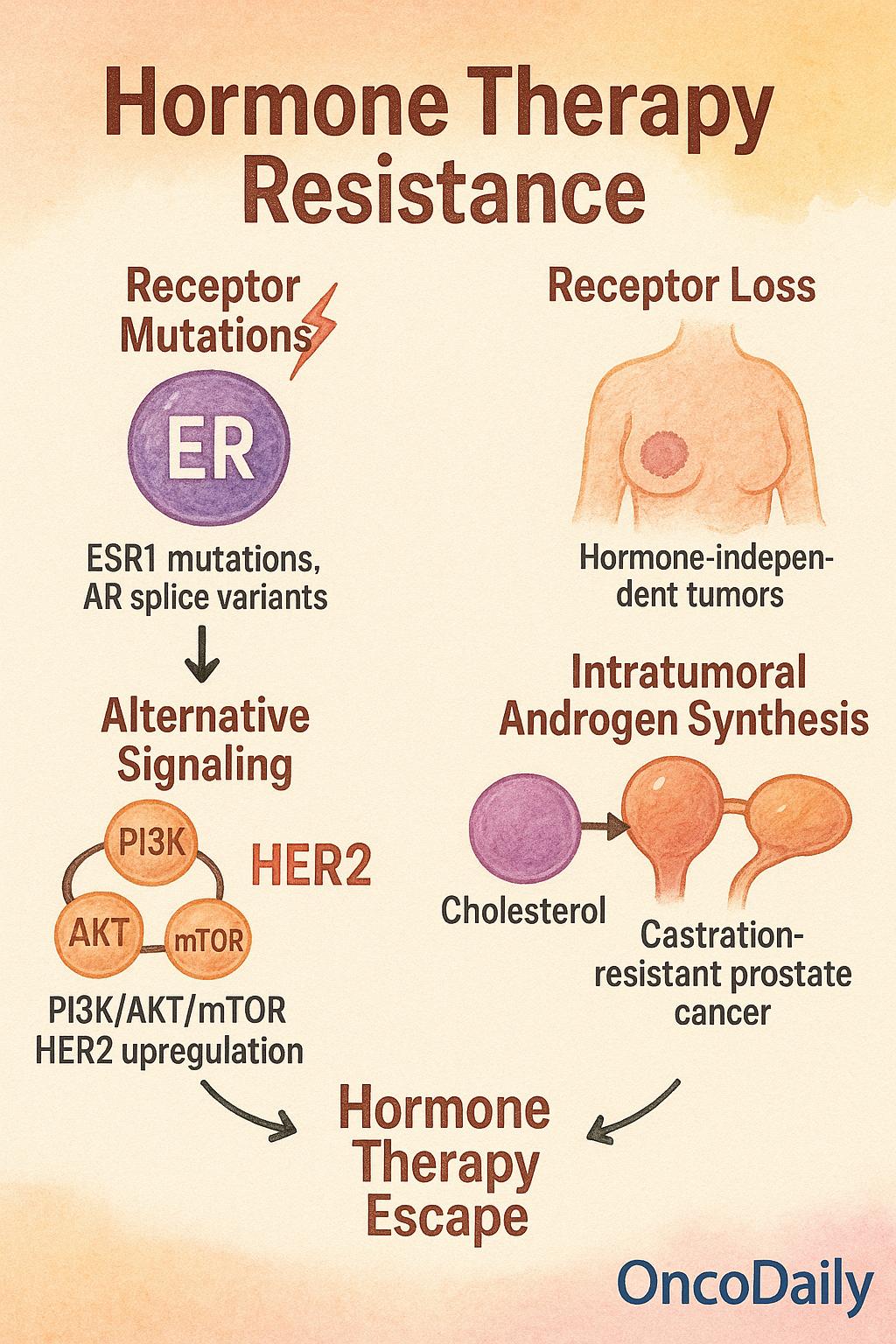
Despite the clinical success of hormonal therapy in managing hormone-dependent cancers, resistance inevitably emerges in a substantial subset of patients. This resistance may be intrinsic (present before treatment) or acquired (developing over time), and it reflects the tumor’s ability to adapt to hormonal pressure through diverse biological mechanisms. Understanding these pathways is essential for optimizing treatment strategies and overcoming therapeutic failure.
One of the most well-characterized mechanisms is the emergence of receptor mutations, particularly in metastatic breast cancer. ESR1 mutations, which alter the ligand-binding domain of the estrogen receptor, can confer constitutive receptor activation, rendering tumors resistant to aromatase inhibitors that rely on estrogen depletion. These mutations are rare in primary tumors but become increasingly prevalent under selective pressure in the metastatic setting. Similarly, in prostate cancer, androgen receptor (AR) splice variants, such as AR-V7, result in truncated, ligand-independent receptors that remain transcriptionally active even in the absence of androgens, contributing to resistance against AR-targeted therapies.
Another common resistance mechanism involves receptor loss or downregulation. Tumors may undergo phenotypic changes during therapy, losing expression of ER or AR entirely and thereby becoming hormone-independent. This phenomenon is more frequently observed in metastatic lesions than in primary tumors, where selective pressure favors clonal expansion of receptor-negative cells. Loss of receptor expression is often associated with a shift toward more aggressive, less differentiated phenotypes.
Alternative signaling pathway activation represents a bypass mechanism whereby tumors shift dependence from hormone receptors to growth factor signaling cascades. Key pathways implicated include the PI3K/AKT/mTOR axis and HER2 upregulation, both of which can sustain proliferation and survival independently of hormone signaling. Crosstalk between these pathways and hormone receptors may also contribute to partial resistance, making combination therapy a rational approach in resistant disease.
In prostate cancer, an additional resistance mechanism involves intratumoral androgen synthesis. Even after systemic androgen deprivation, prostate tumors can locally produce androgens from cholesterol precursors, maintaining AR activation in a hormone-deprived environment. This intracrine mechanism is particularly relevant in castration-resistant prostate cancer (CRPC), where tumor cells exploit steroidogenic enzymes such as CYP17A1 to generate sufficient ligand to sustain AR-driven transcription.
These resistance mechanisms differ markedly between primary and metastatic disease. While primary tumors are often highly hormone-dependent and responsive to therapy, metastatic clones are typically more heterogeneous and possess evolved mechanisms of escape. This dynamic process of adaptation is referred to as “hormone therapy escape”, wherein tumors initially suppressed by hormonal treatment gradually rewire their biology to circumvent dependence on the targeted pathway.
Emerging Strategies and Combination Approaches in Hormonal Therapy
The landscape of hormonal therapy is rapidly evolving, driven by the emergence of novel agents and rational combination strategies designed to overcome resistance and improve efficacy. These innovations reflect a shift from traditional endocrine monotherapy toward more personalized and biomarker-informed treatment approaches.
Among the most promising developments are oral selective estrogen receptor degraders (SERDs), such as elacestrantand giredestrant, which offer improved receptor degradation and bioavailability compared to intramuscular fulvestrant. These agents are particularly useful in tumors harboring ESR1 mutations, a known mechanism of resistance to aromatase inhibitors. In parallel, proteolysis-targeting chimeras (PROTACs) represent a new therapeutic platform that facilitates targeted degradation of hormone receptors via the ubiquitin-proteasome system. In the context of prostate cancer, selective androgen receptor degraders (SARDs) are being developed to target resistant AR variants, including those that evade inhibition by current therapies.
In addition to these novel agents, combination regimens have redefined the standard of care in several settings. In hormone receptor–positive breast cancer, combining hormonal therapy with CDK4/6 inhibitors such as palbociclib, ribociclib, or abemaciclib has significantly improved progression-free and overall survival in both early and metastatic disease. These inhibitors arrest the cell cycle downstream of ER signaling, delaying the emergence of resistance. Similarly, in patients with PIK3CA-mutated tumors, the addition of PI3K inhibitors like alpelisib to endocrine therapy provides a targeted approach to overcoming pathway-driven resistance. mTOR inhibitors, such as everolimus, offer another strategy by suppressing compensatory survival signaling activated upon estrogen deprivation.
In prostate cancer, the concept of triplet therapy has gained momentum, especially in metastatic castration-sensitive disease. This approach involves androgen deprivation therapy (ADT) combined with AR pathway inhibitors (e.g., apalutamide, enzalutamide) and docetaxel chemotherapy. Clinical trials have demonstrated a survival benefit in patients with high-volume disease, marking a significant advancement in upfront treatment intensity.
These evolving strategies are increasingly guided by biomarker stratification, enabling more precise therapeutic selection. Genomic profiling of tumors—such as identifying ESR1 or PIK3CA mutations in breast cancer or AR-V7 expression in prostate cancer—allows clinicians to tailor hormonal therapy based on molecular vulnerabilities. This personalized approach maximizes efficacy while minimizing unnecessary exposure to ineffective treatments.
Toxicities, Long-Term Implications, and Supportive Care in Hormonal Therapy
While hormonal therapies are generally more tolerable than cytotoxic chemotherapy, they are associated with distinct class-specific toxicities that can significantly affect patient well-being, especially with prolonged use. These adverse effects arise from systemic hormone suppression or receptor blockade and often mirror the physiological roles of the targeted hormones in non-malignant tissues.
Estrogen blockade, whether through selective estrogen receptor modulators (SERMs) or SERDs, commonly induces vasomotor symptoms such as hot flashes and night sweats. Tamoxifen, a SERM, also carries a risk of endometrial hyperplasia and carcinoma, particularly in postmenopausal women, due to its partial agonist effect on uterine tissue. Long-term estrogen suppression leads to bone demineralization, increasing the risk of osteopenia and osteoporosis, especially in patients not receiving concurrent bone-protective agents.
Aromatase inhibitors, which deplete estrogen levels in postmenopausal women, are associated with a distinct profile of musculoskeletal toxicity, including arthralgia, myalgia, and stiffness, which can impair adherence. Additionally, prolonged estrogen deprivation may contribute to increased cardiovascular risk, including hyperlipidemia and hypertension, due to estrogen’s protective effects on vascular function and lipid metabolism.
In androgen deprivation therapy (ADT) for prostate cancer, the side effect profile reflects the wide-ranging physiological roles of testosterone. Common toxicities include sexual dysfunction, loss of libido, erectile dysfunction, and reduced muscle mass. ADT also predisposes patients to metabolic syndrome, characterized by insulin resistance, increased abdominal fat, and dyslipidemia, which elevates the risk of cardiovascular disease. Emerging evidence also links long-term ADT to cognitive decline and mood disturbances, raising concerns about quality of life in older patients.
The long-term implications of hormonal therapy necessitate proactive monitoring and supportive care strategies. Bone density should be regularly assessed using DEXA scans, especially in patients receiving AIs or GnRH analogs. Calcium and vitamin D supplementation, along with bisphosphonates or denosumab, may be indicated to preserve skeletal integrity. Mood and cognitive function should be evaluated periodically, particularly in men on ADT and women with severe menopausal symptoms. Cardiovascular risk should be assessed at baseline and during treatment, with management of hypertension, hyperlipidemia, and glucose intolerance as needed.
Future Directions in Hormonal Therapy: Innovation, Integration, and Equity
The future of hormonal therapy in oncology lies in expanding its therapeutic reach, overcoming resistance, and ensuring equitable access to care. Ongoing drug development efforts are moving beyond traditional receptor-targeted agents such as selective estrogen and androgen receptor degraders (SERDs and SARDs), toward next-generation molecules that engage multiple oncogenic drivers simultaneously. These include dual-targeting agents that combine hormonal blockade with inhibition of key survival pathways—such as ER degraders fused with PI3K or CDK4/6 inhibitory domains—to address tumors with complex signaling dependencies.
A critical frontier in this evolution is the exploration of endocrine-tumor-immune interactions, particularly the role of sex hormones in shaping the tumor microenvironment (TME). Emerging evidence suggests that estrogen and androgen signaling can modulate immune cell infiltration, inflammation, and immune evasion mechanisms. Understanding how hormonal therapies affect the TME—and how this can be leveraged to enhance responses to immunotherapy—is a rapidly expanding area of research with the potential to redefine combination treatment strategies.
At the same time, managing hormonal resistance without compromising endocrine health remains a pressing challenge. Prolonged hormone deprivation, particularly in younger patients, can lead to significant long-term sequelae including cardiovascular disease, osteoporosis, metabolic dysfunction, and impaired quality of life. Future strategies must balance the intensity of hormonal suppression with protective interventions that preserve bone, cardiovascular, and cognitive health. There is also a need for validated biomarkers to identify patients who can safely de-escalate or discontinue endocrine therapy after prolonged disease control.
Equally important is the global imperative to bridge access gaps, especially in low- and middle-income countries (LMICs). Despite the cost-effectiveness of many endocrine agents, availability, affordability, and infrastructure limitations often prevent widespread adoption of guideline-recommended hormonal therapies. Newer agents such as oral SERDs or CDK4/6 inhibitors remain out of reach for many populations. Addressing these disparities requires not only pricing and policy reforms but also global investment in healthcare delivery systems, diagnostic capacity, and clinician training.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is hormonal therapy in cancer treatment?
It’s a targeted treatment that blocks hormones like estrogen or testosterone to slow or stop cancer growth.
Which cancers are treated with hormonal therapy?
Mainly breast, prostate, endometrial, and some ovarian cancers.
How does hormonal therapy work in breast cancer?
It blocks estrogen receptors or reduces estrogen production to slow tumor growth in ER+ cancers.
What is androgen deprivation therapy (ADT)?
ADT lowers testosterone levels to treat prostate cancer by slowing its growth.
What are SERDs and how are they used?
Selective Estrogen Receptor Degraders degrade ERs and are used in resistant breast cancer cases.
What are common side effects of hormonal therapy?
Hot flashes, fatigue, bone loss, sexual dysfunction, and cardiovascular risk.
How does cancer become resistant to hormonal therapy?
Through receptor mutations, receptor loss, alternative pathway activation, or intratumoral hormone production.
Can hormonal therapy be combined with other treatments?
Yes, it's often combined with CDK4/6 inhibitors, chemotherapy, or immunotherapy.
Is hormonal therapy used in early-stage or advanced cancer?
It’s used in both early and metastatic stages depending on cancer type and patient status.
Are newer hormonal therapies available?
Yes, newer oral SERDs, SARDs, and combination regimens are under development and clinical use.


