Toripalimab (Loqtorzi): Uses in Cancer, Side Effects, Dosage, Expectations, and More
Toripalimab is a PD-1 inhibitor, a type of immune checkpoint inhibitor that works by blocking the PD-1 pathway, which cancer cells use to evade the immune system. By inhibiting PD-1, Toripalimab helps the immune system recognize and attack cancer cells.
It was first approved in China in 2018 to treat unresectable or metastatic melanoma. In 2023, Toripalimab received FDA approval for use in nasopharyngeal carcinoma (NPC), initially in combination with cisplatin and gemcitabine as first-line therapy for recurrent or metastatic NPC. It was also approved as monotherapy for patients with NPC who have progressed after platinum-based chemotherapy.
Which company produced Toripalimab?
Toripalimab (Loqtorzi) was developed by Junshi Biosciences, a Chinese biopharmaceutical company founded in 2012. The company focuses on developing innovative biologics for cancer, autoimmune diseases, and other conditions. Known for its work in immuno-oncology, Junshi Biosciences has a strong pipeline, including PD-1 inhibitors like Toripalimab, one of China’s first domestically developed PD-1 therapies.
Junshi formed key partnerships with Coherus BioSciences and AstraZeneca to expand its global reach. Coherus received rights to develop and commercialize Toripalimab in North America, while AstraZeneca handles promotion in select indications within China. These strategic alliances support the global advancement of this innovative cancer immunotherapy.
How does Toripalimab work?
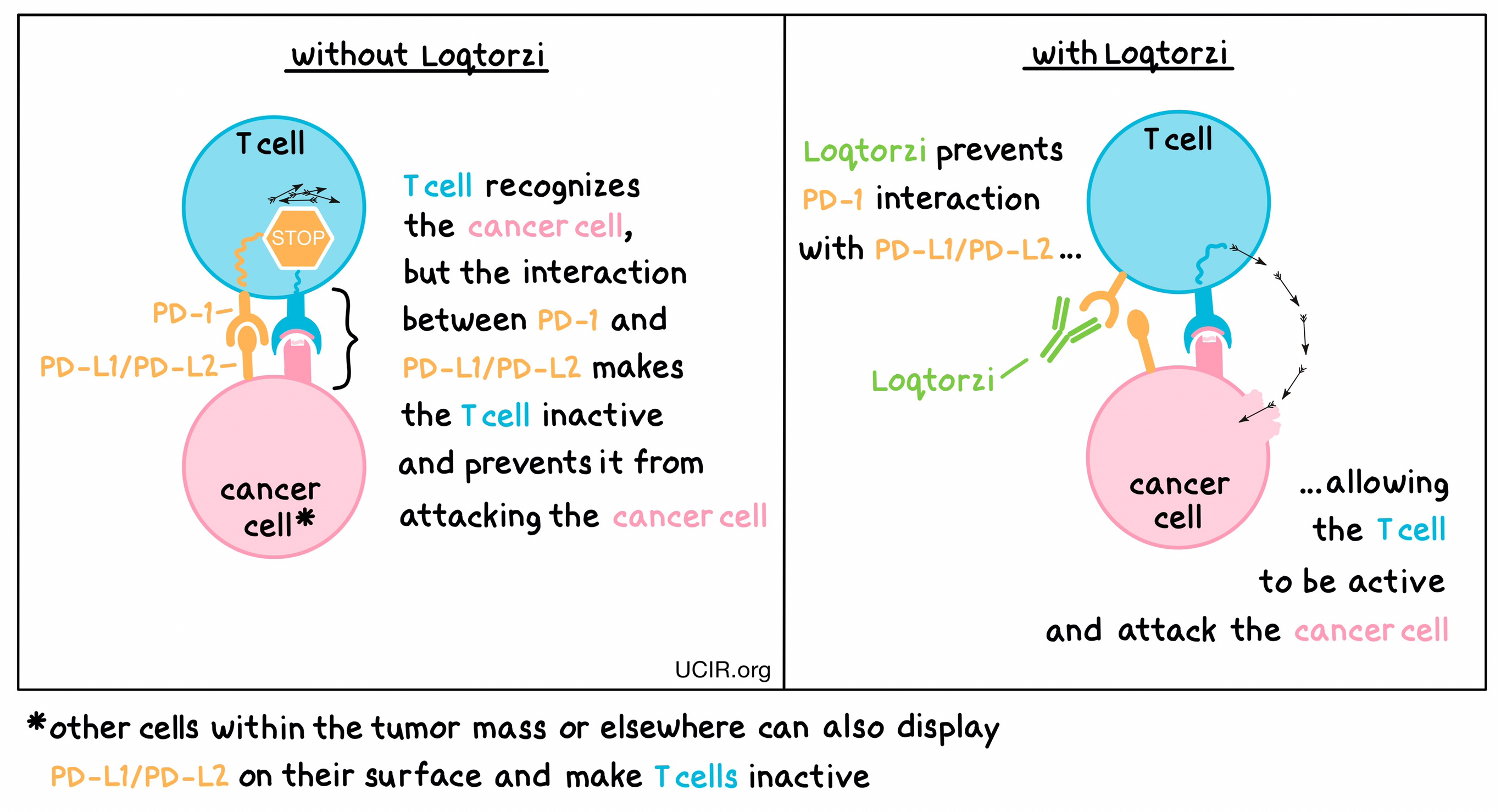
Toripalimab is a PD-1 (programmed cell death protein 1) inhibitor, a type of immunotherapy designed to enhance the body’s immune response against cancer.
Normally, the immune system uses T-cells to detect and destroy abnormal or cancerous cells. However, cancer cells can develop mechanisms to evade immune detection. One such mechanism involves the PD-1 pathway. PD-1 is a checkpoint protein on T-cells that, when activated by its ligands (PD-L1/PD-L2) on tumor cells, sends a signal to turn off the T-cells, preventing them from attacking the cancer.
Toripalimab works by blocking the interaction between PD-1 and its ligands. By inhibiting this pathway, Toripalimab prevents the “off” signal, allowing T-cells to stay active and continue targeting and destroying cancer cells.
What Cancers Is Toripalimab Approved to Treat?
Loqtorzi was first approved in China in December 2018 for metastatic melanoma. It was later approved for the treatment of nasopharyngeal carcinoma (NPC). In October 2023, the FDA approved Toripalimab for use in the U.S. to treat NPC, both in combination with chemotherapy as a first-line treatment and as a monotherapy for patients with disease progression after platinum-based chemotherapy.
These approvals highlight Toripalimab’s growing role in cancer immunotherapy, particularly for challenging cancers like melanoma and NPC.
What research is behind the approval?
The approvals of Loqtorzi by the U.S. Food and Drug Administration (FDA) and China’s National Medical Products Administration (NMPA) were primarily based on the outcomes of two pivotal clinical trials:
JUPITER-02 Study
The JUPITER-02 trial has established Loqtorzi plus gemcitabine-cisplatin (GP) as a new standard of care for recurrent/metastatic nasopharyngeal carcinoma (NPC). The 2021 JCO publication showed that Toripalimab significantly improved progression-free survival (PFS) over GP alone (11.7 vs. 8.0 months, HR = 0.52, p = 0.0003) and increased response rates (77.4% vs. 66.4%).
The final overall survival (OS) results, published in JCO in May 2023, confirmed a clinically significant OS benefit. By November 2022, with 30.1 months median follow-up, OS was not reached in the toripalimab arm versus 33.7 months in the placebo arm (HR = 0.63, p = 0.0083). The 3-year OS rates were 64.5% vs. 49.2%, favoring Loqtorzi across all subgroups.
While safety remained manageable, immune-related adverse events were more frequent (54.1% vs. 21.7%). These findings reinforce toripalimab plus GP as a superior first-line treatment for r/m NPC.
POLARIS-02 Study
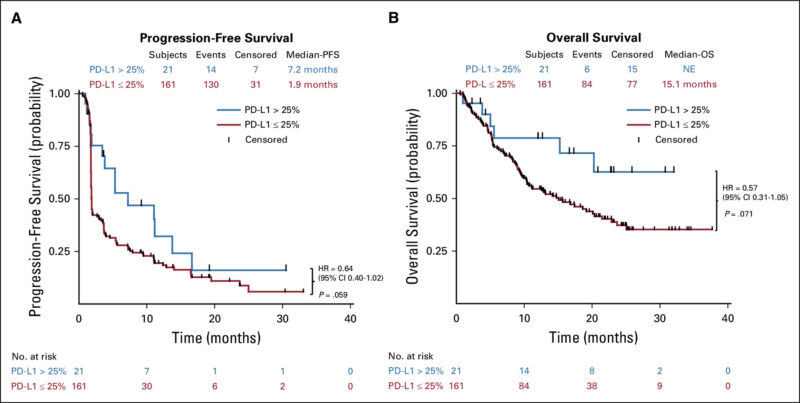
The POLARIS-02 trial, published in JCO on January 25, 2021, evaluated toripalimab in pretreated recurrent/metastatic nasopharyngeal carcinoma (NPC). In this single-arm, phase II study, 190 patients received Loqtorzi 3 mg/kg every two weeks until disease progression or unacceptable toxicity.
Results showed an objective response rate (ORR) of 20.5%, with a median duration of response (DOR) of 12.8 months, progression-free survival (PFS) of 1.9 months, and overall survival (OS) of 17.4 months. Among patients who had failed at least two prior lines of chemotherapy, the ORR was 23.9%. Responses were similar regardless of PD-L1 status, but a ≥50% drop in plasma Epstein-Barr virus (EBV) DNA at day 28 correlated with better outcomes (ORR 48.3% vs. 5.7%, p = .0001).
The study confirmed toripalimab’s durable response and manageable safety in chemorefractory NPC, with early EBV DNA decline as a potential predictive biomarker.
Toripalimab Combinations and Treatment Outcomes
Loqtorzi is primarily used with other therapies for enhanced treatment outcomes, particularly in cancer immunotherapy. Below are some of the combinations and their corresponding treatment outcomes:
Toripalimab + Chemotherapy (Cisplatin and Gemcitabine)
Toripalimab, combined with cisplatin and gemcitabine, is approved for first-line nasopharyngeal carcinoma (NPC) treatment. The JUPITER-02 study demonstrated that this combination significantly improved progression-free survival (PFS) compared to chemotherapy alone. Patients receiving toripalimab had a higher response rate, offering a more effective option for metastatic or recurrent NPC.
Toripalimab as Monotherapy
Toripalimab is approved as monotherapy for recurrent or metastatic nasopharyngeal carcinoma (NPC) after progression on platinum-based chemotherapy. The POLARIS-02 study showed that toripalimab provided clinical benefits with a manageable safety profile, making it an effective second-line treatment for patients with limited options.
Toripalimab + Other Targeted Therapies
A phase II study published in Annals of Oncology (Dec 2023) evaluated neoadjuvant treatment with Toripalimab plus Axitinib for resectable mucosal melanoma. The combination therapy achieved a 33.3% pathological response rate, including 4 patients with a complete response. Responders had a median recurrence-free survival of 11.7 months.
The treatment increased CD3+/CD8+ T cell infiltration, suggesting an enhanced immune response. These promising results support further research into this combination for improving survival in mucosal melanoma, a more aggressive and harder-to-treat subtype.
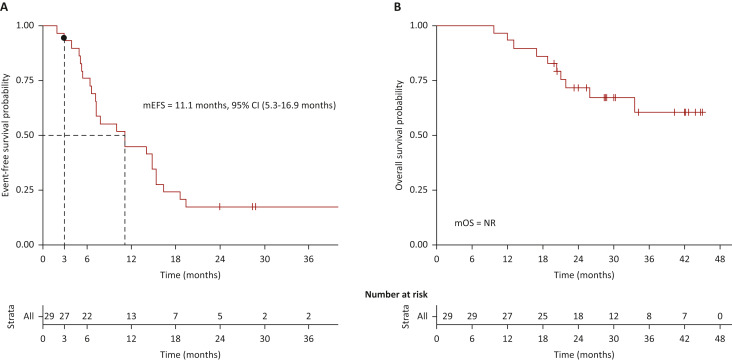
A phase II trial published in Springer Nature Link (Feb 1, 2025) evaluated the combination of surufatinib and toripalimab in treatment-naive advanced or metastatic PD-L1-positive non-small-cell lung cancer (NSCLC) and previously treated small-cell lung cancer (SCLC). The treatment showed promising efficacy, with an objective response rate (ORR) of 57.1% in the NSCLC cohort and 15.8% in the SCLC cohort.
The median progression-free survival (PFS) was 9.6 months for NSCLC and 3.0 months for SCLC, while the median overall survival (OS) was 24.3 months for NSCLC and 11.0 months for SCLC. Treatment was generally well-tolerated, with 55.8% of patients experiencing grade ≥3 treatment-related adverse events. These results suggest that the combination offers encouraging antitumor activity and a manageable safety profile.
Toripalimab’s role in combination therapies, particularly with chemotherapy or as monotherapy after chemotherapy failure, has made it a vital treatment option in cancer immunotherapy, showing promising survival benefits and improving treatment outcomes for patients with advanced cancers.
Toripalimab side effects and its management
Loqtorzi is a powerful immunotherapy used to treat certain cancers, such as nasopharyngeal carcinoma (NPC). While it offers significant benefits in improving survival and outcomes for cancer patients, like all medications, it comes with potential side effects that require careful monitoring and management.
Common side effects
Patients receiving Loqtorzi may experience several common side effects. The most frequent is fatigue, along with hypothyroidism (low thyroid levels), musculoskeletal pain, and gastrointestinal issues such as nausea and constipation. Skin reactions like rashes, fever, and peripheral neuropathy (tingling or pain in the limbs) are also commonly reported. Additionally, some may experience respiratory symptoms (coughing, upper respiratory infections), headaches, dizziness, and general malaise.
Serious Side Effects
More serious, immune-related side effects can occur with Toripalimab. These include pneumonitis (lung inflammation causing breathing issues), colitis (abdominal pain and diarrhea), and hepatitis (liver inflammation). Endocrine problems such as hypophysitis (pituitary gland inflammation) and other endocrinopathies can also arise. Nephritis (kidney inflammation), severe skin reactions like blistering, and myocarditis (heart inflammation) require immediate attention. In rare cases, neurological toxicities such as confusion or seizures can occur.
Management of Side Effects
Managing Toripalimab’s side effects involves regular monitoring of thyroid, liver, kidney function, and blood counts. If immune-related side effects occur, doctors may administer steroids or other immunosuppressive agents. In cases of severe reactions, treatment may be paused or doses adjusted.
Patient education is crucial, ensuring individuals know when to report any new symptoms to their healthcare provider for timely intervention.
By closely monitoring and managing these side effects, healthcare providers can help patients get the most out of their treatment while minimizing risks.
What is the Recommended Dosage of Toripalimab?
Loqtorzi is available as an injectable solution, 40 mg/mL in a 6-mL single-dose vial.
Nasopharyngeal Carcinoma (NPC)
For the first-line treatment of metastatic or recurrent NPC, Loqtorzi is given in combination with cisplatin and gemcitabine at a dose of 240 mg IV every 3 weeks, continuing until disease progression, unacceptable toxicity, or for up to 24 months. When used as monotherapy for recurrent or metastatic NPC after platinum-based chemotherapy, the recommended dose is 3 mg/kg IV every 2 weeks, continuing until disease progression or unacceptable toxicity.
Read more about Immunotherapy vs Chemotherapy: Success Rates, Side Effects, Costs Compared on OncoDaily.
How is Toripalimab administered?
Toripalimab is compatible with 0.9% NaCl and should be administered using polypropylene infusion bags and a 0.2- or 0.22-micron inline filter. The vial should be inspected for particulate matter and discoloration before use. If the solution is clear to slightly yellow with no visible particles, the required volume is withdrawn and injected into a 100 mL or 250 mL NaCl infusion bag. The solution should be gently inverted to mix but not shaken. The final concentration must be 1-3 mg/mL.
For IV administration, the infusion pump should be used with a 0.2- or 0.22-micron inline filter. The first infusion should take at least 60 minutes, and subsequent infusions can be shortened to 30 minutes if no reactions occur. Toripalimab should not be coadministered with other drugs via the same IV line. If given alongside chemotherapy, Loqtorzi should be administered first.
Storage: Unopened vials should be stored at 2-8°C (36-46°F), away from light. If not used immediately, diluted solutions can be stored at room temperature for 8 hours or refrigerated for 24 hours. Any unused solution after this time should be discarded, and the solution should never be frozen.
What to Avoid During Toripalimab Treatment?
During Loqtorzi treatment, it’s important to avoid coadministering certain antibiotics, such as cefepime, ceftriaxone, ceftazidime/avibactam, cefotetan, cefazolin, cefaclor, and oxacillin, as they may decrease the effectiveness of Loqtorzi. This interaction could interfere with the therapeutic effects of immune checkpoint inhibitors. It’s advisable to consult with a healthcare provider before starting any new medications during Toripalimab therapy.
Read more about Targeted Therapy vs Immunotherapy: Which Is Right for You, Key Differences & Similarities on OncoDaily.
Toripalimab effectiveness over time
Loqtorzi has shown effectiveness over time in clinical trials, particularly for nasopharyngeal carcinoma (NPC) and advanced solid tumors. In combination with cisplatin and gemcitabine, it improved overall survival and progression-free survival in first-line treatment. As monotherapy, it also showed benefits for patients with disease progression after platinum-based chemotherapy.
Long-term use of Toripalimab has led to durable responses in some patients, but immune-related adverse events can impact treatment continuation. Ongoing clinical trials aim to enhance its long-term effectiveness and minimize toxicity.
Ongoing trials with Toripalimab
The Phase II trial (NCT06823427) is evaluating the efficacy of 9MW2821 + Toripalimab versus 9MW2821 monotherapy in patients with locally advanced or metastatic urothelial carcinoma who have not received prior systemic treatment in this setting.
The Phase Ib trial (NCT06882876) is evaluating the safety and efficacy of JS014 + Toripalimab + TACE in patients with unresectable hepatocellular carcinoma (HCC). The primary endpoint is safety and tolerability, while secondary endpoints include ORR, DCR, TTP, PFS, and OS. This study explores how combining immunotherapy with TACE can enhance anti-tumor immune responses.
The Phase II trial (NCT06843889) is evaluating Toripalimab + Neoadjuvant Chemoradiotherapy in locally advanced esophageal squamous cell carcinoma. Thirty-four patients received Loqtorzi with radiotherapy, followed by surgery after 4–6 weeks. The study assesses pCR, MPR, DFS, OS, and survival rates, along with treatment safety.
FAQ
How does Toripalimab work in the body?
Toripalimab is a PD-1 inhibitor that helps the immune system recognize and attack cancer cells by blocking the PD-1 pathway, which tumors use to evade immune responses.
How is Loqtorzi different from other immunotherapy drugs?
While Toripalimab works similarly to other PD-1 inhibitors like pembrolizumab and nivolumab, it is specifically approved for NPC and is being explored in multiple clinical trials for other cancers.
How is Toripalimab administered?
Loqtorzi is given as an intravenous (IV) infusion over 30–60 minutes, typically every two or three weeks, depending on the specific treatment plan.
Are there any lifestyle changes needed during Toripalimab treatment?
Patients should avoid alcohol, live vaccines, and certain antibiotics (such as cefepime and ceftriaxone). A balanced diet, hydration, and rest can help manage side effects.
Can Toripalimab be used in combination with other cancer treatments?
Yes, Loqtorzi is often combined with chemotherapy, radiation therapy, or other targeted treatments, depending on the type of cancer being treated.
Are there ongoing clinical trials for Toripalimab?
Yes, Toripalimab is being studied in clinical trials for other cancers, including urothelial carcinoma, hepatocellular carcinoma (HCC), and esophageal squamous cell carcinoma.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
