DESTINY-Breast06 trial data presented at the ESMO Breast Cancer 2025 Congress in Munich, May 14, 2025 further solidify trastuzumab deruxtecan (T-DXd) as a preferred post-endocrine therapy option in patients with hormone receptor-positive (HR+), HER2-low and HER2-ultralow metastatic breast cancer, marking a pivotal advance in the HER2-targeted therapeutic continuum.

Image Taken from the DESTINY-Breast06 Phase III Trial Presentation at ESMO Breast Cancer 2025
Trial Design and Clinical Context
DESTINY-Breast06 was a multicenter, open-label, phase 3 trial that enrolled 866 patients with HR+ metastatic breast cancer characterized as HER2-low (IHC 1+ or 2+/ISH−) or HER2-ultralow (IHC 0 with faint membrane staining). These patients were chemotherapy-naïve in the metastatic setting and had received:
- At least two lines of endocrine therapy (with or without targeted therapy), or
- One line of endocrine therapy and progression within six months of starting endocrine + CDK4/6 inhibitor treatment, or
- Recurrence within 24 months of adjuvant endocrine therapy.
Patients were randomized 1:1 to receive T-DXd (5.4 mg/kg IV every 3 weeks) or treatment of physician’s choice (TPC) — either capecitabine (59.8%) or a taxane (40.2%). The primary endpoint was progression-free survival (PFS) in the overall intention-to-treat (ITT) population, with secondary endpoints including overall response rate (ORR), duration of response (DOR), PFS2 (progression-free survival on next-line therapy), and safety.
Efficacy Outcomes
The primary analysis had already shown a 5.1-month PFS improvement in the ITT population favouring T-DXd: Median PFS:
- T-DXd: 13.3 months
- TPC: 8.2 months
- HR: 0.64 (95% CI: 0.54–0.76; P < .001)
At ESMO 2025, further subgroup analyses were presented, showing that T-DXd consistently outperformed both chemotherapy agents:
Objective Response Rate (ORR)
- T-DXd vs Capecitabine: 57.9% vs 30.7%
- T-DXd vs Taxane: 56.5% vs 31.8%
Duration of Response (DOR)
- T-DXd vs Capecitabine: 14.6 months vs 11.4 months
- T-DXd vs Taxane: 13.7 months vs 6.2 months
PFS2 (Progression-Free Survival after Next-Line Therapy)
- T-DXd vs Capecitabine: 22.6 vs 16.4 months (HR: 0.62; 95% CI: 0.49–0.78)
- T-DXd vs Taxane: 18.2 vs 13.6 months (HR: 0.61; 95% CI: 0.47–0.80)
These results highlight not only the initial benefit of T-DXd but also its potential to extend disease control beyond first progression, a key consideration in HR+ metastatic disease.
Regulatory Impact
Based on earlier DESTINY-Breast06 findings, the U.S. FDA approved T-DXd in January 2025 for the treatment of unresectable or metastatic HR+/HER2-low or ultralow breast cancer following endocrine resistance. This marked the first regulatory recognition of HER2-ultralow as a therapeutic target. At the same time, the Ventana PATHWAY anti-HER2 (4B5) assay was approved as a companion diagnostic for detecting HER2-ultralow tumors.
Safety Profile
Adverse events were consistent with previous reports. T-DXd showed a manageable safety profile, with certain toxicities more frequent compared to chemotherapy but generally offset by longer treatment durations.
Any-grade TRAEs (T-DXd vs Capecitabine):
- Nausea: 68.7% vs 28.1%
- Fatigue: 48.7% vs 34.5%
- Neutropenia: 35.1% vs 17.7%
- Vomiting: 25.3% vs 10.8%
- Palmar-plantar erythrodysesthesia: 0.4% vs 53.4%
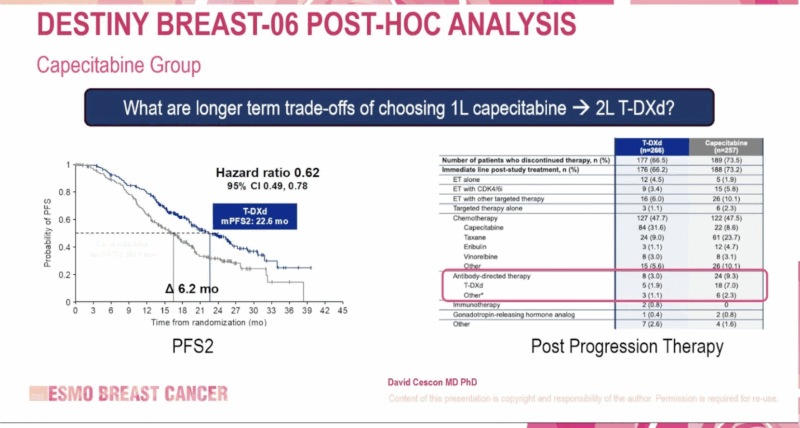
Image Taken from the DESTINY-Breast06 Phase III Trial Presentation at ESMO Breast Cancer 2025
Any-grade TRAEs (T-DXd vs Taxane):
- Nausea: 61.5% vs 16.7%
- Fatigue: 43.8% vs 33.9%
- Neutropenia: 41.4% vs 42.3%
- Vomiting: 30.2% vs 7.1%
- Peripheral sensory neuropathy: 1.8% vs 20.8%
Barrios highlighted that neutropenia was the most common grade ≥3 TRAE with both T-DXd and taxanes, while hand-foot syndrome was predominant with capecitabine.
“When adjusted for treatment duration, the overall safety profile of T-DXd was generally similar to or better than that of capecitabine or taxane,” Barrios concluded.
Expert Perspective
“When adjusted for treatment duration, the overall safety profile of T-DXd was generally similar to or better than that of capecitabine or taxane,” Barrios stated. “These findings further support T-DXd as an effective treatment option in HR+/HER2-low or ultralow metastatic breast cancer after at least one line of endocrine therapy.”
He concluded, “With improvements in PFS, response rate, and durability—and a predictable, manageable toxicity profile—T-DXd is redefining how we approach HER2-expressing breast cancers, even at the lowest levels.”
Discussion
The DESTINY-Breast06 trial represents a turning point in the management of hormone receptor-positive, HER2-low and HER2-ultralow metastatic breast cancer. The results presented at ESMO Breast Cancer 2025 not only confirm the statistically significant and clinically meaningful benefit of T-DXd over traditional chemotherapy but also validate HER2 expression as a therapeutic continuum, rather than a binary biomarker.
Historically, patients with HER2-low or ultralow tumors were considered HER2-negative and excluded from HER2-targeted therapies. This study challenges that paradigm. The consistent superiority of T-DXd in terms of PFS, ORR, DOR, and PFS2 across both HER2-low and ultralow subgroups confirms that even minimal HER2 expression is sufficient for T-DXd activity. Importantly, the benefits were observed regardless of the comparator—whether capecitabine or taxane—highlighting the robustness of the drug’s efficacy.
Safety remains an essential consideration in metastatic settings, where long-term disease control and quality of life are equally important. T-DXd’s safety profile was consistent and manageable, with a tolerability profile that compares favorably to standard chemotherapy when adjusted for duration of exposure. Notably, neurotoxicity and hand-foot syndrome—common limiting factors for taxanes and capecitabine, respectively—were less prominent with T-DXd.
The FDA’s decision to approve T-DXd for HER2-ultralow breast cancer, alongside the expanded use of companion diagnostics, marks a regulatory milestone. This trial has helped define HER2-ultralow as a clinically actionable subgroup, expanding treatment options for thousands of patients worldwide.
What They’re Saying: Reactions to EMBER-3 at ESMO Breast 2025
Elia Seguí, MD,Breast Medical Oncologist at MD Anderson Cancer Center, shared on X
Loved the discussion led by @DavidCescon at #ESMOBreast2025! Fully agree that for patients with access to T-DXd in 2L, capecitabine remains a very appealing 1L option—especially in the absence of rapid symptomatic progression.
Also Read About EMBER-3 Trial Updates on Oncodaily
Disclosures
Dr. Barrios reported consulting fees and honoraria from AstraZeneca, Daiichi Sankyo, Lilly, MSD, Novartis, Pfizer, Roche/Genentech, and others. He also holds equity in MedSIR and Tummi.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


