The U.S. Food and Drug Administration (FDA) has provided positive regulatory feedback supporting the continued clinical development of eftilagimod alfa (efti) in combination with Keytruda® (pembrolizumab) for the first-line treatment of patients with recurrent or metastatic head and neck squamous cell carcinoma (1L HNSCC) who have PD-L1 expression levels below 1% (CPS <1). These patients, who currently lack chemotherapy-free options, represent a subgroup with high unmet clinical need.
The feedback is based on results from the Phase IIb TACTI-003 trial, which demonstrated the potential of this novel immunotherapy combination to induce meaningful clinical responses even in tumors with low or absent PD-L1 expression. The update was shared via an official press release published on Immutep’s website, the company developing efti.
TACTI-003 Trial: Exploring an Unmet Need
The TACTI-003 (KEYNOTE-PNC-34) trial is a Phase IIb, multicenter, open-label study investigating the efficacy and safety of efti in combination with pembrolizumab in patients with previously untreated, recurrent or metastatic HNSCC. The trial enrolled 171 patients across more than 30 clinical sites in the United States, Europe, and Australia.
Patients were divided into two main cohorts based on PD-L1 expression levels.
- Cohort A (CPS ≥1) was randomized to receive either the efti-Keytruda combination or pembrolizumab alone.
- Cohort B (CPS <1) received the combination treatment only, as monotherapy is not approved for this group.
Efti was administered subcutaneously at a dose of 30 mg every 2 or 3 weeks, while pembrolizumab was given intravenously at 400 mg every 6 weeks. The trial’s primary endpoint was Overall Response Rate (ORR) assessed by RECIST 1.1, with secondary endpoints including Progression-Free Survival, Overall Survival, and Duration of Response.

Learn more about Head and Neck Cancer: Hidden Risks, Mutations and Outcomes on OncoDaily.
Clinical Results: Strong Response Across All PD-L1 Levels
Topline results from the trial, initially reported in June 2024 and further updated in July during an ESMO Virtual Plenary session, showed that the combination of efti and pembrolizumab consistently outperformed pembrolizumab alone across all PD-L1 subgroups.
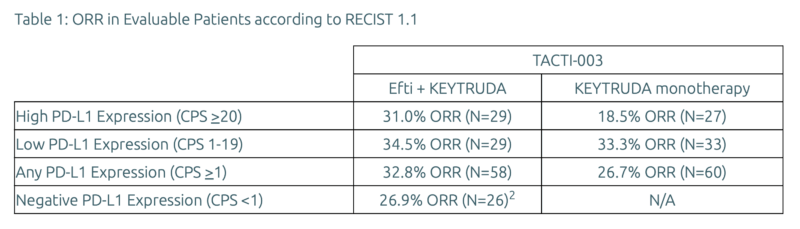
In the randomized Cohort A, patients with high PD-L1 expression (CPS >20) achieved an ORR of 31.0% with the combination therapy, compared to 18.5% with pembrolizumab alone. Among those with lower PD-L1 expression (CPS 1–19), the ORR was 34.5% with the combination versus 33.3% with pembrolizumab alone. Overall, patients with any level of PD-L1 expression (CPS ≥1) had an ORR of 32.8% on the combination therapy compared to 26.7% on monotherapy.
In Cohort B, which included patients with PD-L1–negative tumors (CPS <1), the response rate reached 26.9%, a significant result in a population where effective immunotherapy options are lacking.
Source: Immutep Official Website
Safety Profile
Eftilagimod alfa (efti) combined with pembrolizumab maintains a favorable safety profile with no new safety signals. In Cohort B of the TACTI-003 Phase IIb trial, 31 patients with recurrent or metastatic head and neck squamous cell carcinoma (PD-L1 CPS <1) received efti (30 mg subcutaneously every 2-3 weeks) plus pembrolizumab (400 mg IV every 6 weeks). The combination achieved a clinically meaningful objective response rate (ORR) of 35.5% and a disease control rate (DCR) of 58.1%, including nearly 10% complete responses, regardless of HPV status.
The treatment was well tolerated without significant added toxicity or increased immune-related adverse events compared to historical data. Dr. Frédéric Triebel, CSO of Immutep, highlighted the durable responses driven by efti’s unique activation of dendritic cells and the adaptive and innate immune system. Despite the relatively small patient numbers, the combination showed a ~68% improvement in the largest patient group with high PD-L1 expression (CPS >20) in a randomized setting.
About Eftilagimod Alfa (Efti)
Eftilagimod alfa (efti) is a first-in-class MHC Class II agonist and soluble LAG-3 fusion protein developed by Immutep, designed to activate both the innate and adaptive immune systems. By binding to MHC Class II molecules on antigen-presenting cells, efti promotes the activation of CD8+ cytotoxic T cells, CD4+ helper T cells, natural killer (NK) cells, and monocytes. This mechanism of action enhances immune system activation in a way that is distinct yet complementary to immune checkpoint inhibitors like pembrolizumab.
The drug has demonstrated a favorable safety profile and is currently being explored in various solid tumors, including HNSCC, non-small cell lung cancer (NSCLC), and metastatic breast cancer. Efti has received Fast Track designation from the FDA for both first-line HNSCC and NSCLC, further validating its clinical potential.
Key Takeaways
- The FDA supports further development of the efti and pembrolizumab combination for PD-L1 negative head and neck cancer patients, a group with limited treatment options.
- The TACTI-003 trial demonstrated improved response rates across all PD-L1 levels, including a 26.9% ORR in PD-L1 negative patients.
- Efti’s unique mechanism as an MHC Class II agonist activates the immune system differently from checkpoint inhibitors, potentially expanding immunotherapy benefits to more patients.
- The combination shows a favorable safety profile with durable responses.


