Roger Li, Urologic oncologist at Moffitt Cancer Center, shared on X/Twitter:
“Final results of CORE001 trial out now at Nature Medicine.
35 patients with BCG-UR CIS treated with Creto + pembro.
- 12months CR (per RBB) = 57.1% (ITT)
- 24months CR = 54% *(Auth Corr)
- No patient progressed to MIBC
- No synergistic toxicity.

Patient population resembles that of typical BCG-UR CIS population
– Median age = 72
– 94.3% male
– Vast majority from the USA
– 77.1% pure CIS.
What is truly remarkable was NOT ONLY the high response rate, BUT the long-term durability of respons
– CR at 3months ~83%
– CR at 24months 54%* (Auth Corr)
After all, patients are looking to spare their bladders long-term.

Equally important: NO pt had progressive disease, including 7 who underwent RC (pT0, pTaLG, pTaHG x 2, pTis x 3); 1 patient died of non-cancer related comorbidity.
Outside of cystectomy, probably the best strategy to prevent missing disease progression and the opportunity for cure.
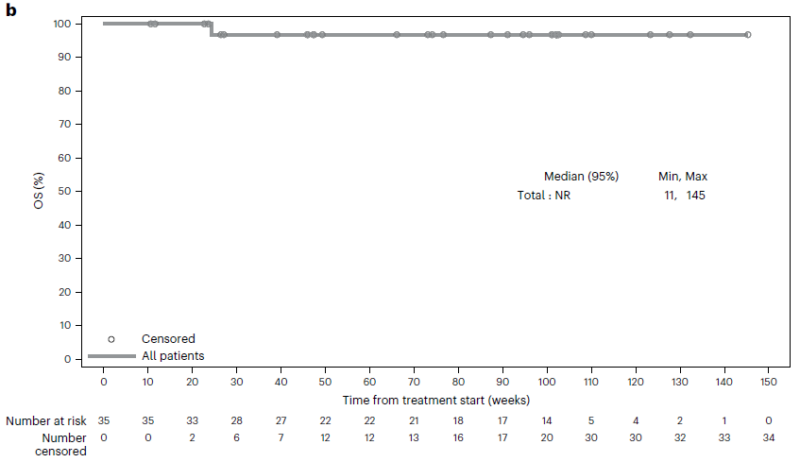
Looked at in that light, AE profile not too bad: no synergistic toxicity, most AEs grade 1-2 bladder related symptoms, 14.3% Gr3 AE.
Why it worked
– Residual CIS served as neoantigen burden
– Intravesical infusion (unlike intratumoral inj) of creto exposed ALL lesions to treatment
– Combo applied in a setting of limited disease burden (as opposed to the metastatic setting in TVEC + Pembro melanoma study).
Our study provides optionality based on patient preference:
1. Fearful of disease progression, and tolerant of ~14% SAE – Creto + pembro
2. Fearful of SAE, tolerant of small chance of disease progression – Creto vs. approved agent.
More to come from IBCG.
So many people to thank, but first the patients and their families for putting their trust in us.
I’d like to dedicate this study to all who has suffered complications from RC for disease refractory to BCG, of whom I know many so dearly options.
Thank you to CG Oncology for your unwavering support; and to Merck for your collaboration.
Much more to come as we look at the translational correlates to understand how it actually worked.
James burke, Arthur Kuan
It’s been a long time coming since we said ‘We gonna shock the world!’ with this study at SITC2021. We did it!!!

Professor Gary Steinberg – Thank you for being a staunch ally of the program through the thick and thin, and your mentorship throughout this entire process.
Co-I’s Paras Shah, Tyler Stewart, Trinity J. Bivalacqua, Don Lamm, Ed Uchio, Dan Geynisman, Joe Jacob, Joshua Meeks, Rian Dickstein Shane Pearce, Ashish M. Kamat, Pat Keegan and Nataliya Hnat colleagues; trial coordinators, pharmacists, RNs, etc – couldn’t have done it without each and every one of you!
Nature Medicine – Kudos to a spectacular editorial experience with the expedited review and publication, truly unparalleled!
Saheli Sadanand and Joao Monteiro thanks for shepharding us through across the finish line!
More to come as we unpack the MoA of the drug James Mule, Jose Conejo-Garcia, Patrick Hwu, Patrick Hwu, Moffitt Urologic Oncology Fellowship, Moffitt Cancer Center.
Onto BOND003 with Mark Tyson et al.”
Source: Roger Li/X
