Zhaohui Su, VP of Biostatistics at Ontada, shared a post on LinkedIn by Massoud Toussi, Real-World Evidence and Epidemiology Leader at Toussilver and Editor-in-Chief at Epidemiology Open Access Journal, adding:
“Target Trial Emulation (TTE) and real-world data (RWD) quality are critical for producing trustworthy regulatory-grade evidence.
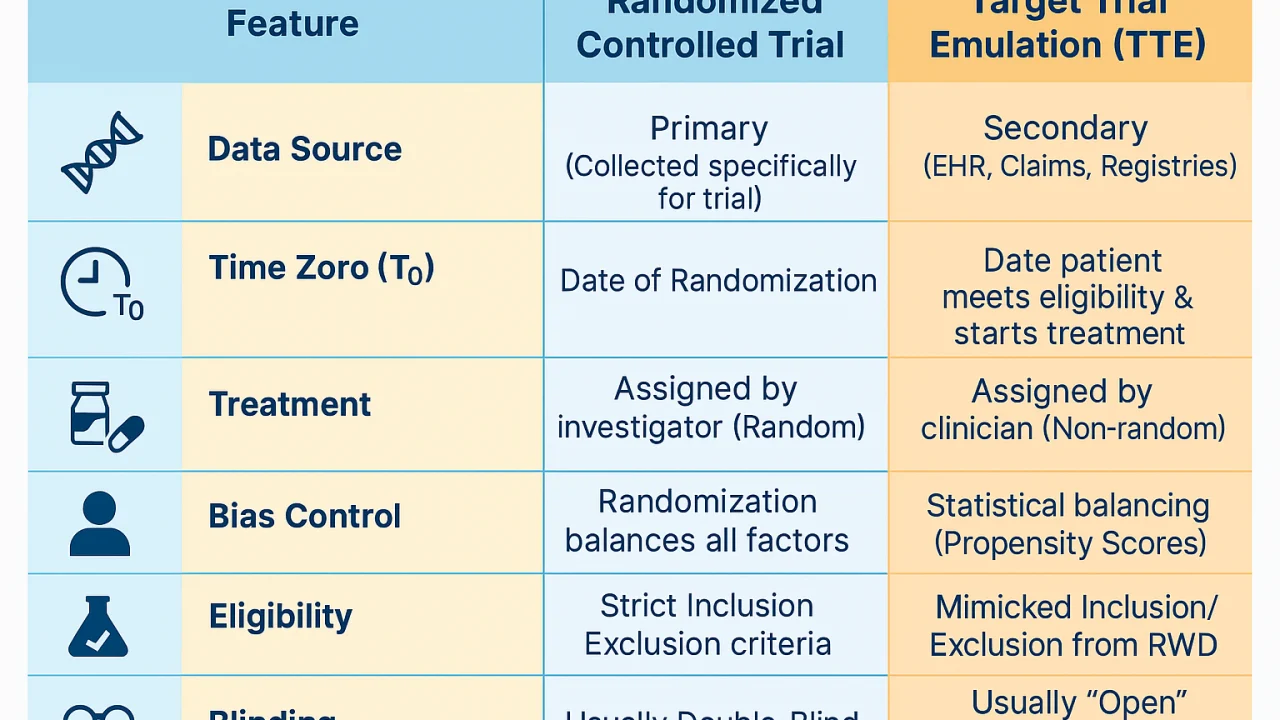
TTE provides a structured framework that applies randomized trial principles to observational studies, reducing biases by aligning time zero, eligibility criteria, and treatment assignment while using advanced statistical methods to emulate randomization.
TTE combined with high-quality RWD elevates real-world evidence (RWE) from simple observation to robust, actionable insights that meet regulatory and clinical standards.”
Quoting Massoud Toussi’s post:
“Is Real-World Evidence (RWE) finally overcoming its reputation for being ‘biased’?
For years, the gold standard for the ascertainment of efficacy has been the Randomized Controlled Trial (RCT). But we are entering a new era where we can ’emulate’ those trials using existing data—if we use the right framework.
I just published a deep dive into Target Trial Emulation (TTE). In the article, I break down:
- Why ‘Time Zero’ is the most common mistake in RWE.
- How the new 2025 TARGET guidelines are changing the game.
- Why the FDA is now looking for this specific methodology in submissions.
If you’re in Clinical Research, HEOR, or Data Science, this is a must-read.
Why ‘Target Trial Emulation’ is the Future of Regulatory-Grade RWE.”
More posts featuring Zhaohui Su.


