Massoud Toussi, Real-World Evidence and Epidemiology Leader at Toussilver and Editor-in-Chief at Epidemiology Open Access Journal, shared a post on LinkedIn:
“Is Real-World Evidence (RWE) finally overcoming its reputation for being ‘biased’?
For years, the gold standard for the ascertainment of efficacy has been the Randomized Controlled Trial (RCT). But we are entering a new era where we can ’emulate’ those trials using existing data—if we use the right framework.
I just published a deep dive into Target Trial Emulation (TTE). In the article, I break down:
- Why ‘Time Zero’ is the most common mistake in RWE.
- How the new 2025 TARGET guidelines are changing the game.
- Why the FDA is now looking for this specific methodology in submissions.
If you’re in Clinical Research, HEOR, or Data Science, this is a must-read.
Why ‘Target Trial Emulation’ is the Future of Regulatory-Grade RWE
Introduction
When talking about the use of Real-World Evidence (RWE) for the ascertainment of efficacy, the biggest criticism has always been bias. I insist on this, as many clinicians and decision makers falsely think that RWE is always inferior to Randomised Clinical Trials (RCTs).
This is not true, and in a lot of situations, RWE is superior or the only answer (for example, take the measure of adherence). Back to the measure of efficacy, real-world data is criticized for being messy, non-randomized, and prone to ‘confounding by indication.’ But what if we could design observational studies using the same rigorous logic as a clinical trial?
The Target Trial Emulation (TTE) is a framework that helps us turning ‘messy data’ into ‘regulatory-grade evidence.’
What exactly is Target Trial Emulation?
At its core, TTE is a conceptual framework popularized by researchers like Miguel Hernán at the Harvard CAUSALab. The principle is simple but profound:
‘To analyze your observational data correctly, you must first imagine the ideal randomized trial you wish you could have conducted to answer your question.’
By explicitly defining the ‘Target Trial’ first, you create a blueprint that prevents the most common errors in RWE, such as immortal time bias and selection bias.
The 3 Pillars of a Successful TTE
If you are reading an RWE paper or designing a study, these three elements are the ‘hints’ that TTE is being used correctly:
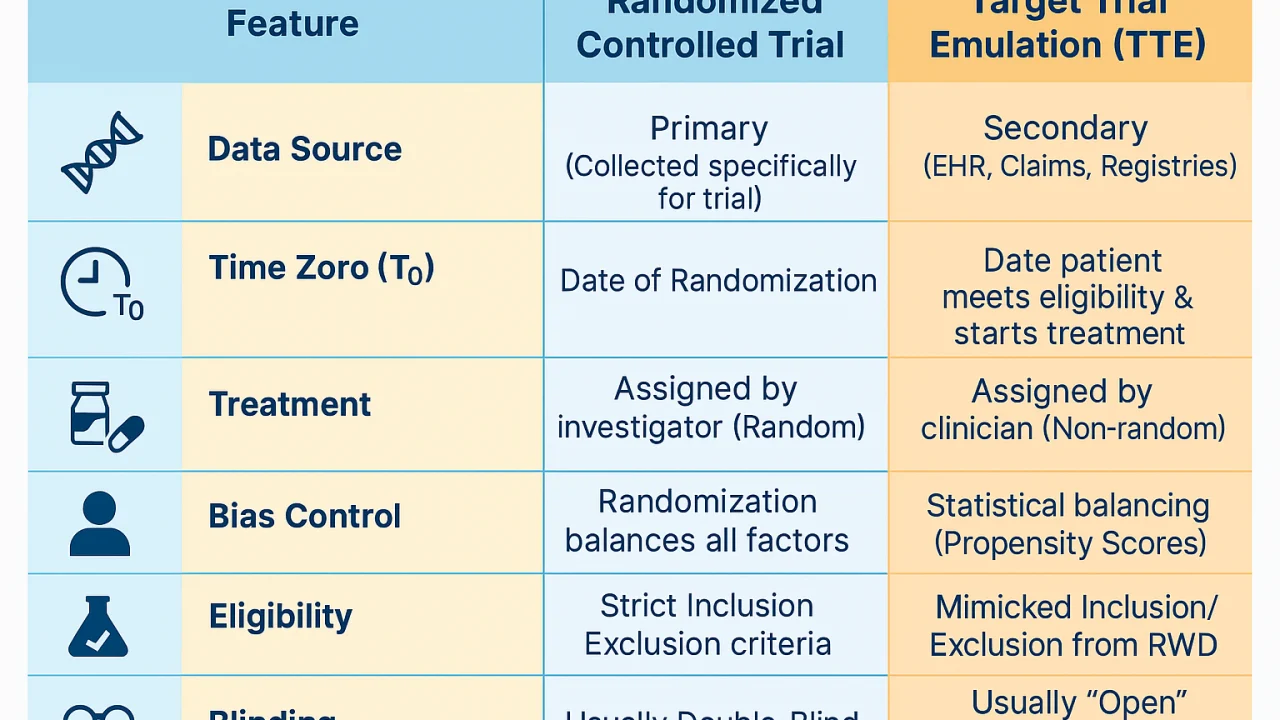
1. The ‘Zero Time’ (T0)
In an RCT, ‘Time Zero’ is the moment of randomization. In many poor RWE studies, researchers accidentally include data from before a patient started a drug. TTE forces you to align the start of follow-up, the eligibility criteria, and the treatment assignment at the exact same moment.
2. Eligibility Criteria
You must apply the same ‘Inclusion/Exclusion’ criteria to your database that you would in a clinic. If a patient wouldn’t have qualified for the trial (e.g., they have a contraindication), they shouldn’t be in your RWE analysis. Again, we are talking about an efficacy trial. In a safety study, this would be a crime!
3. Emulating Randomization (Causal Inference)
Since we can’t actually randomize patients in the past, we use advanced statistics—like Propensity Score Matching or Inverse Probability Weighting or other methods (see my past posts) —to ‘balance’ the groups so they look as similar as possible, effectively mimicking a coin flip.
Why is the Industry Obsessed with TTE Right Now?
The FDA and EMA are increasingly mentioning TTE in their guidance documents. The reason? Reproducibility.
- The GLP-1 Example (see my next post): Recent studies in JAMA used TTE to show heart failure benefits in GLP-1 users. Because they emulated the ‘STEP-HFpEF’ trials, the results were far more convincing to clinicians.
- New Reporting Standards: In September 2025, the TARGET guidelines were published in JAMA, establishing a mandatory checklist for any researcher claiming to use this framework.
Conclusion: From ‘Observation’ to ‘Causality’
The shift from ‘descriptive RWE’ to ‘causal RWE’ is the most important trend in drug development today. By using Target Trial Emulation, we aren’t just looking at what happened; we are understanding why it happened.
For professionals in the life sciences, mastering the language of TTE is no longer optional—it’s the new standard for evidence.
Resources and Further Reading (Copy and Paste as Links)
- The Methodology: Hernán MA, Robins JM (2016). Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available.
- New Reporting Standards (2025): The TARGET Guideline for Studies Emulating a Target Trial.
- Educational Hub: Harvard CAUSALab (The pioneers of the TTE framework).”
You can also read more posts on Real-World Evidence on OncoDaily.


