Sergio Cifuentes Canaval, Medical Oncologist at Las Américas Auna Clinic, shared a post on LinkedIn:
“Adjuvant CDK4/6 Inhibitors in HR+/HER2- Early Breast Cancer: A Debate Still Open.
The role of CDK4/6 inhibitors in the adjuvant setting for hormone receptor-positive, HER2-negative early breast cancer remains one of the most active and polarizing discussions in breast oncology. Two recent perspectives published in The Lancet Oncology present strong arguments on both sides – highlighting how much we still need to learn before this approach becomes standard for all patients.
The case for recommending adjuvant CDK4/6i:
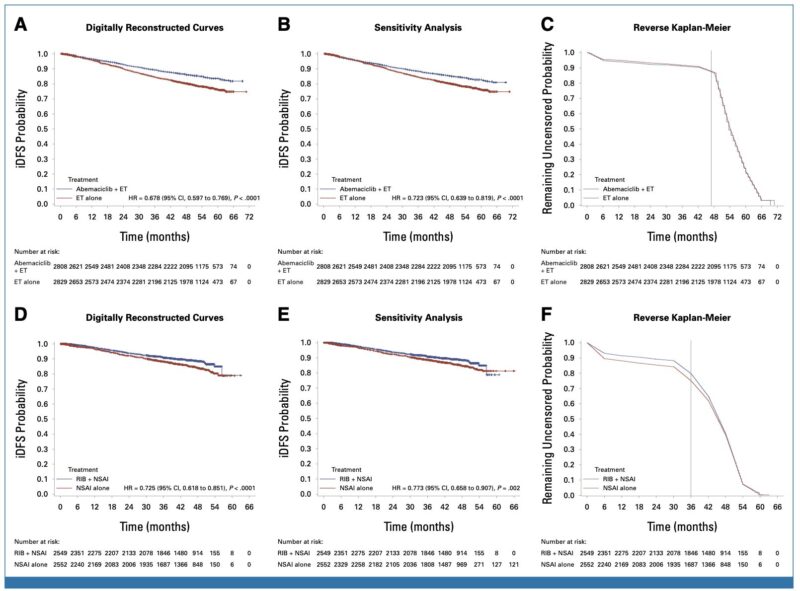
Trials such as monarchE (abemaciclib) and NATALEE (ribociclib) demonstrated improvements in invasive disease-free survival (iDFS), especially in high-risk populations.
These benefits are driven largely by a reduction in distant recurrences, with encouraging absolute risk reductions and acceptable numbers needed to treat.
Differences in pharmacology and duration of therapy between agents might explain variability in outcomes.
Importantly, patient-reported quality of life impacts were limited in most domains.
The case against routine use:
Other phase 3 trials, including PALLAS and PENELOPE-B, failed to show benefit.
Toxicity, frequent dose reductions, and treatment discontinuations raise concerns about real-world tolerability.
The high cost and uncertain cost-effectiveness – particularly without proven overall survival benefit – remain major barriers.
OS data are still immature, and trial design limitations (e.g., lack of uniform CDK4/6i access at progression) complicate interpretation.
Potential informative censoring and heterogeneity across studies further cloud the evidence.
Where does this leave us?
The evidence suggests that adjuvant CDK4/6 inhibitors may be beneficial for carefully selected high-risk patients. However, broader use requires more mature survival data, improved patient selection (possibly with biomarkers like ctDNA), and a deeper understanding of cost-effectiveness in different health systems.
Clinical takeaway:
Until we have clearer answers, decision-making should remain highly individualized – weighing risk, toxicity, patient preferences, and system-level factors. The debate highlights the evolving complexity of precision oncology and the importance of multidisciplinary collaboration in defining standards of care.
How are you approaching adjuvant CDK4/6 inhibition in your clinical practice? Are you offering it routinely to high-risk patients, or still waiting for stronger survival data?”
Title: Why Adjuvant Treatment With a CDK4/6 Inhibitor Should Be Recommended for Women With High-Risk Breast Cancer: Methodologic Considerations on Available Evidence
Authors: Fabio Conforti, Federico Merlo, Laura Pala, Jacopo Canzian, Benedetta Tinterri, Tommaso De Pas, Marzia Locatelli, James C. Dickerson, Javier Cortes, Dennis J. Slamon, Richard Gelber, Vincenzo Bagnardi
Read the Full Article in the Journal of Clinical Oncology. 
Title: Why We Do Not Recommend That Women With Breast Cancer Receive Adjuvant Treatment With a CDK4/6 Inhibitor
Authors: Ian F. Tannock, Qamar J. Khan, Tito Fojo
The Full Article.

More posts featuring Sergio Cifuentes Canaval on OncoDaily.


