Masahiro Torasawa, Medical Oncologist at Juntendo University, shared a post on X:
“How to Reduce Skin Toxicity of Amivantamab + Lazertinib? – COCOON Trial (Ph2)
Key Results
- Significantly reduced Gr ≥2 dermatologic AEs within 12 weeks (42% vs 75%, p<0.0001).
- Less impact of skin symptoms on QoL.
- Delayed time to onset of severe skin AEs (median 1.0 mo → 3.5 mo).
- Limited effect on paronychia (21% vs 23%; p=0.76)
Discussion
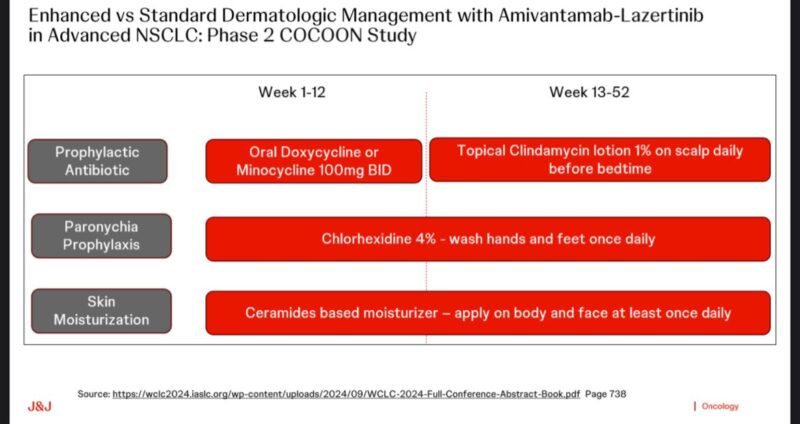
- A simple, widely available prophylactic regimen greatly improves tolerability in clinical practice.
- Essential supportive care to maximize the efficacy benefits seen in the MARIPOSA trial.
- New strategies are needed to manage paronychia.
- Prophylactic anticoagulation for the first 4 months is also effective for VTE risk.
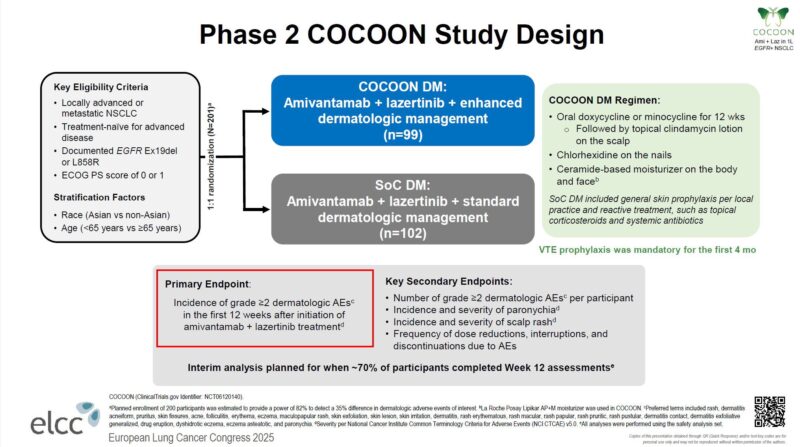
Grade 2 or higher skin or nail adverse events were significantly reduced with the regimen as described.”
Title: Enhanced Versus Standard Dermatologic Management With Amivantamab-Lazertinib in EGFR-Mutated Advanced NSCLC: The COCOON Global Randomized Controlled Trial
Authors: Byoung Chul Cho, Weimin Li, Alexander I. Spira, Maxwell Sauder, Jill Feldman, Farastuk Bozorgmehr, Milena Mak, Janellen Smith, Pei Jye Voon, Baogang Liu, Panwen Tian, Jiunn-Liang Tan, Cheng-Ta Yang, Jin-Yuan Shih, Nuri Karadurmus, Juan Esteban Cundom, Glaucio Bertollo, Irfan Cicin, Jorge Nieva, Ana Laura Ortega-Granados, Pascale Tomasini, Danny Nguyen, Enriqueta Felip, Julia Schuchard, Sean P. Murphy, Bailey G. Anderson, Tonatiuh Romero, Yichuan Xia, Shubin Sheng, Joshua M. Bauml, Parthiv J. Mahadevia, Julian Kam, Mehregan Nematian-Samani, Jairo Simoes, Mark Wildgust, Nicolas Girard
Read the Full Article on Journal of Thoracic Oncology

More posts featuring Masahiro Torasawa.


