Tom Powles, Head of Solid Tumor Research at Barts Cancer Institute, shared a post on X:
“IMVIGOR011 shows ctDNA is a useful predictive and prognostic tool post cystectomy in muscle invasive bladder cancer.
ctDNA positive patients had DFS and OS benifits with atezolizumab.
ctDNA negative patients are at a low risk of relapse/cancer death.
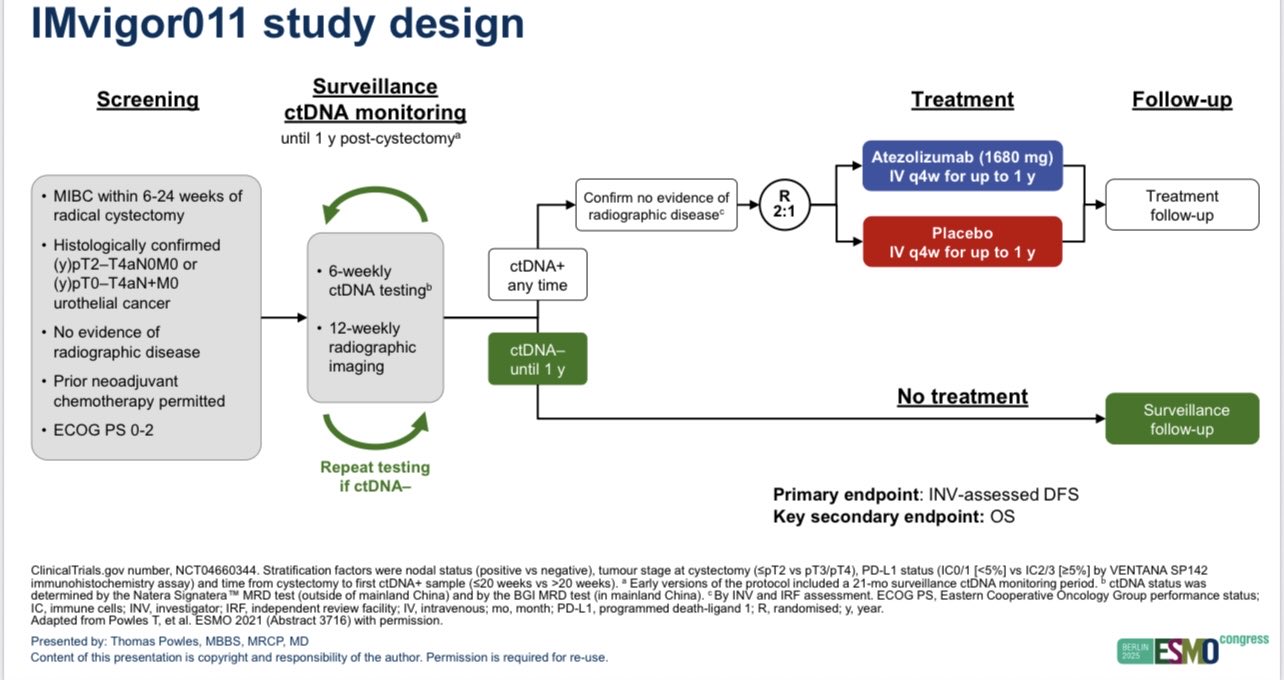
Atezo was associated with 24% ctDNA clearance rate.
Monitoring ctDNA negative patients post cystectomy for a year identified a subgroup of patients who switched from negative to positive who also benefited.
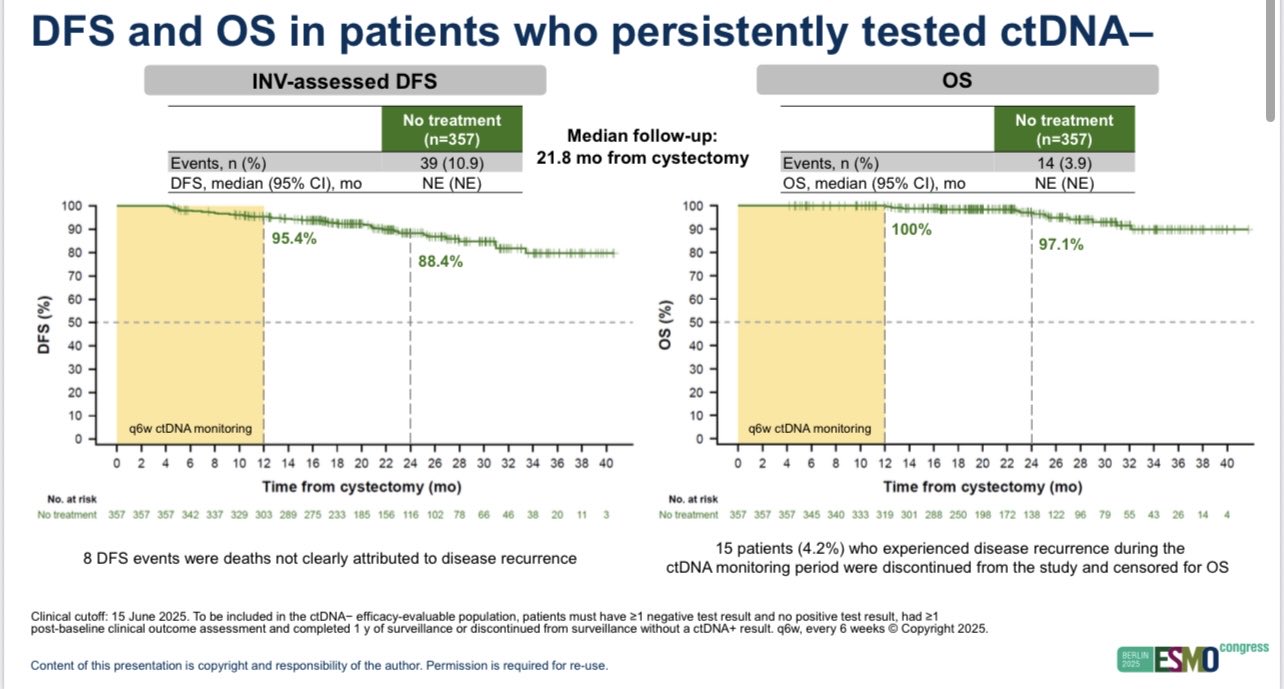
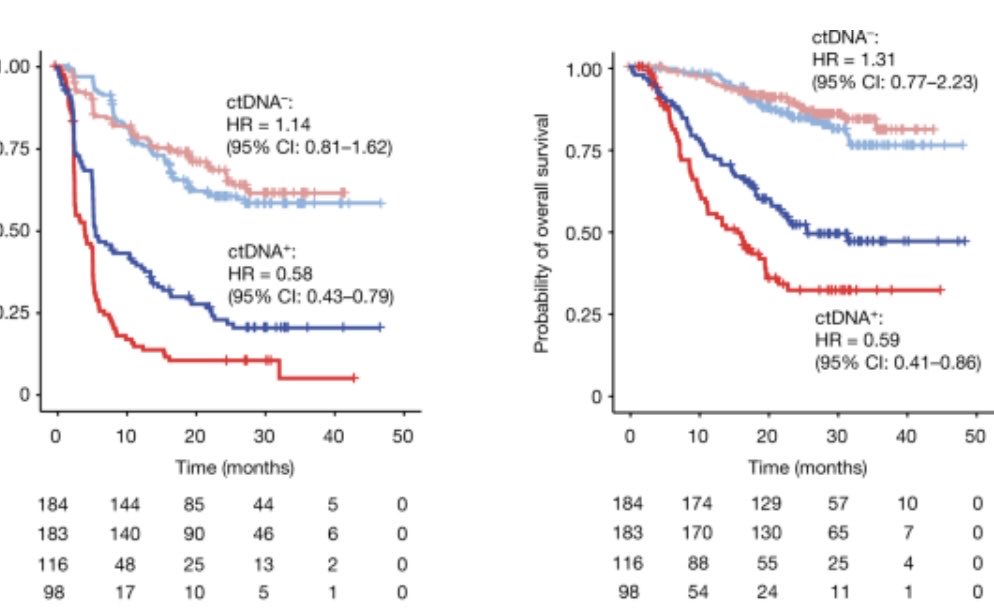
The hypothesis germinated from the negative ImVigor010 trial (see in figure) where we showed only the ctDNA positive subgroup benefited from adjuvant atezolizumab.
The collaboration of pharmaceutical industry, hospital networks precision biotechnology.
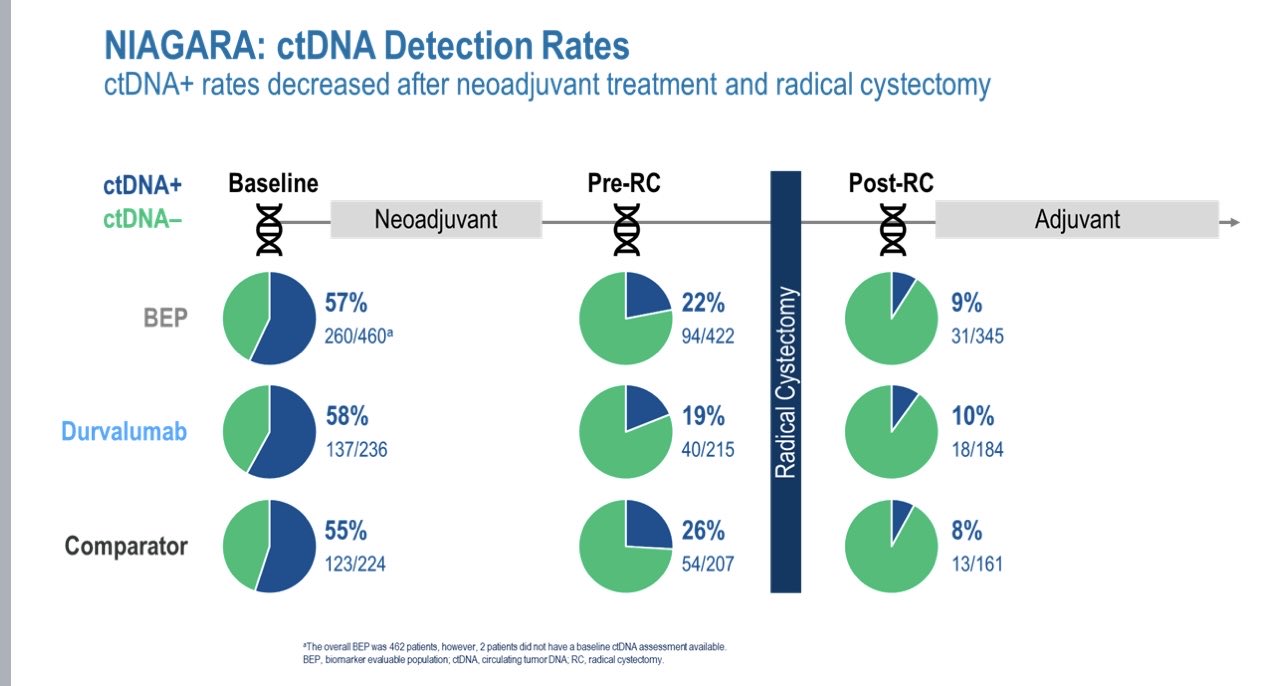
Do these data tell us if we can stop ongoing perioperative immune therapy (NIAGARA KN905)?
Sadly not.
Other randomised trials will be needed to address this.
ctDNA analysis data from NIAGARA is below. All of these data were with the informed signitera assay.”
You can also read:
ESMO 2025 Day 1 Highlights Not to Miss
ESMO 2025 Day 2 Highlights Not to Miss
ESMO 2025 Day 3 Highlights Not to Miss



