Vincent Rajkumar, Professor of Medicine at the Mayo Clinic in Rochester and Editor‑in‑Chief at Blood Cancer Journal, shared a post on X about a paper he co-authored with colleagues published in NEJM:
“Daratumumab is now FDA and EU approved for treatment of high risk smoldering myeloma. It is recommended level 1 by NCCN.
My thoughts: In the AQUILA randomized trial, daratumumab:
-Prolonged time to active myeloma
-Prolonged time to end organ damage
-Delayed time to needing active myeloma therapy
-Improved overall survival
-Improved PFS-2
-Low toxicity
-No detriment to QOL
This is a limited duration (3 years) therapy. This limited duration therapy can prevent or delay full blown typically continuous myeloma therapy by years. Full myeloma therapy is not simple. It involves 4 drug initial therapy, stem cell transplant in eligible patients, and prolonged 1-2 drug maintenance. Delaying time to full myeloma therapy by a long time is in and of itself a big deal. Watching patients closely and starting therapy only when you see evidence of progression sounds good on paper but in reality will lead to more patients starting full myeloma therapy sooner.
It also risks end organ damage between visits. Experts think they can watch carefully but in studies we find progressions are missed at top centers. In real world the type of follow up we did in AQUILA will not be possible. Patients with active myeloma in my experience are struggling to see their doctors in time. We hope to have more overall survival information soon. But we already see an important improvement. For more on the trial see.
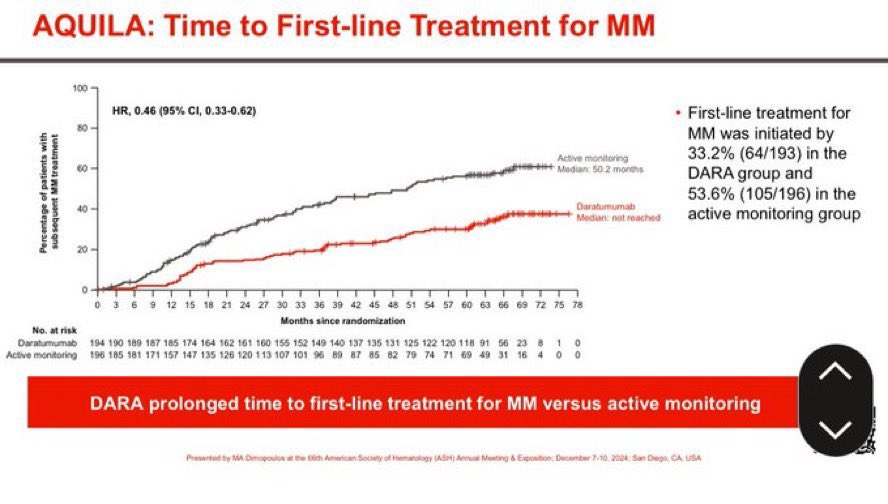
How do we define high risk smoldering myeloma? Typically the best way to interpret a randomized trial is to see if you had a patient today, would they have been considered eligible for the RCT? However, risk stratifications improve and change over time. AQUILA enrolled patients 2017-2019. Mayo developed the 20-2-20 criteria in 2018. The IMWG validated the 20-2-20 risk stratification and this was published in 2020.
Risk stratification will continue to evolve. At a minimum AQUILA results (Daratumumab as an intervention) apply to patients defined as high risk by the Mayo 2018 IMWG 20-2-20 system. (High risk SMM is when any of these 3 are high >20% marrow plasma cells, >2gm/dl M spike, >20 free light chain ratio.
Note: the IMWG cytogenetic model and scoring in the IMWG risk stratification paper is not validated. Also the “high” risk group when adding cytogenetics to the 20-2-20 model is in reality an “ultra high risk group” and the intermediate risk is the high risk group for practice. So if you are using the cytogenetic /scoring model, patients in both intermediate and high risk group will be considered high risk smoldering myeloma for purposes of clinical practice.
While some worry about overtreatment of high risk smoldering myeloma, some others worry about under treatment! They feel that in patients with high risk smoldering myeloma especially with higher levels of marrow involvement it’s better to treat as myeloma. I don’t agree with this approach at the present time. This is a hypothesis that needs to be tested: Do patients with high risk smoldering myeloma have better outcomes with monotherapy (like used in AQUILA or the ECOG trial led by Sagar Lonial) versus myeloma-like therapy. This is being addressed in randomized trials, at least one of which is still accruing. We need to wait for the trials to complete and for data. We have seen attractive hypothesis fail when tested in an RCT. So even though I feel that certain high risk SMM patients may benefit from myeloma like therapy, for now I think for the most part we should wait for more data and discussion.”
Title: Daratumumab or Active Monitoring for High-Risk Smoldering Multiple Myeloma
Authors: Meletios A. Dimopoulos, Peter M. Voorhees, Fredrik Schjesvold, Yael C. Cohen, Vania Hungria, Irwindeep Sandhu, Jindriska Lindsay, Ross I. Baker, Kenshi Suzuki, Hiroshi Kosugi, Mark-David Levin, Meral Beksac, Keith Stockerl-Goldstein, Albert Oriol, Gabor Mikala, Gonzalo Garate, Koen Theunissen, Ivan Spicka, Anne K. Mylin, Sara Bringhen, Katarina Uttervall, Bartosz Pula, Eva Medvedova, Andrew J. Cowan, Philippe Moreau, Maria-Victoria Mateos, Hartmut Goldschmidt, Tahamtan Ahmadi, Linlin Sha, Annelore Cortoos, Eva G. Katz, Els Rousseau, Liang Li, Robyn M. Dennis, Robin Carson, and S. Vincent Rajkumar
You can read the Full Article in The New England Journal of Medicine.
Title: International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM)
Authors: María-Victoria Mateos, Shaji Kumar, Meletios A. Dimopoulos, Verónica González-Calle, Efstathios Kastritis, Roman Hajek, Carlos Fernández De Larrea, Gareth J. Morgan, Giampaolo Merlini, Hartmut Goldschmidt, Catarina Geraldes, Alessandro Gozzetti, Charalampia Kyriakou, Laurent Garderet, Markus Hansson, Elena Zamagni, Dorotea Fantl, Xavier Leleu, Byung-Su Kim, Graça Esteves, Heinz Ludwig, Saad Usmani, Chang-Ki Min, Ming Qi, Jon Ukropec, Brendan M. Weiss, S. Vincent Rajkumar, Brian G. M. Durie & Jesús San-Miguel

You Can Also Read: FDA Approves Daratumumab and Hyaluronidase-fihj for High-Risk Smoldering Multiple Myeloma



