Abel Costa, Hematologist-oncologist at Hospital São Luiz – Rede D’Or São Luiz, shared a post on X:
“Truly grateful and proud to be part of a defining moment at ASH 2025.
Contributing as an author to the EPCORE FL-1 trial — with simultaneous publication in The Lancet and global regulatory impact — was a very special milestone in my academic journey.
Follicular lymphoma remains a chronic disease for most patients, with diminishing benefit after each line of therapy.
EPCORE FL-1 addressed a critical unmet need: a fixed-duration, chemo-free strategy capable of delivering deep and durable remissions.
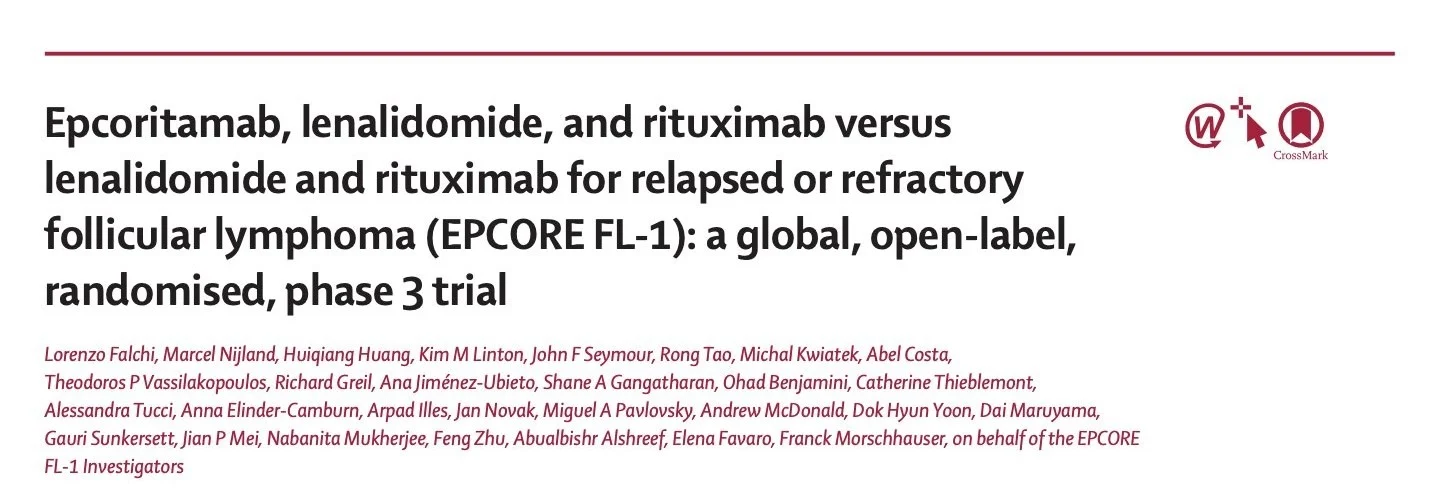
EPCORE FL-1 design:
- Phase 3, global, randomized
- Epcoritamab + R² vs R² • R/R FL after ≥1 prior line
- Dual primary endpoints: ORR and PFS
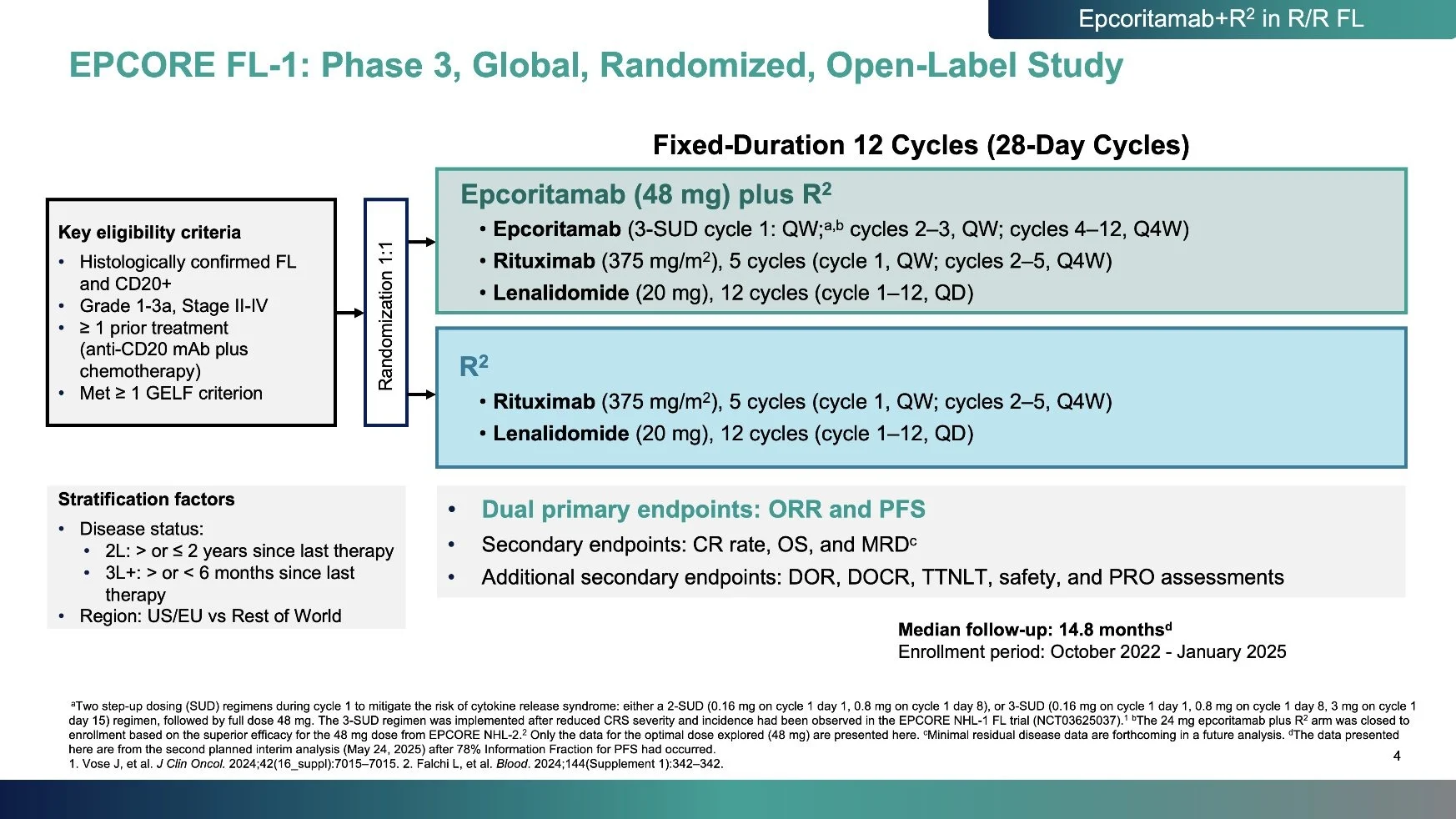
Key efficacy results:
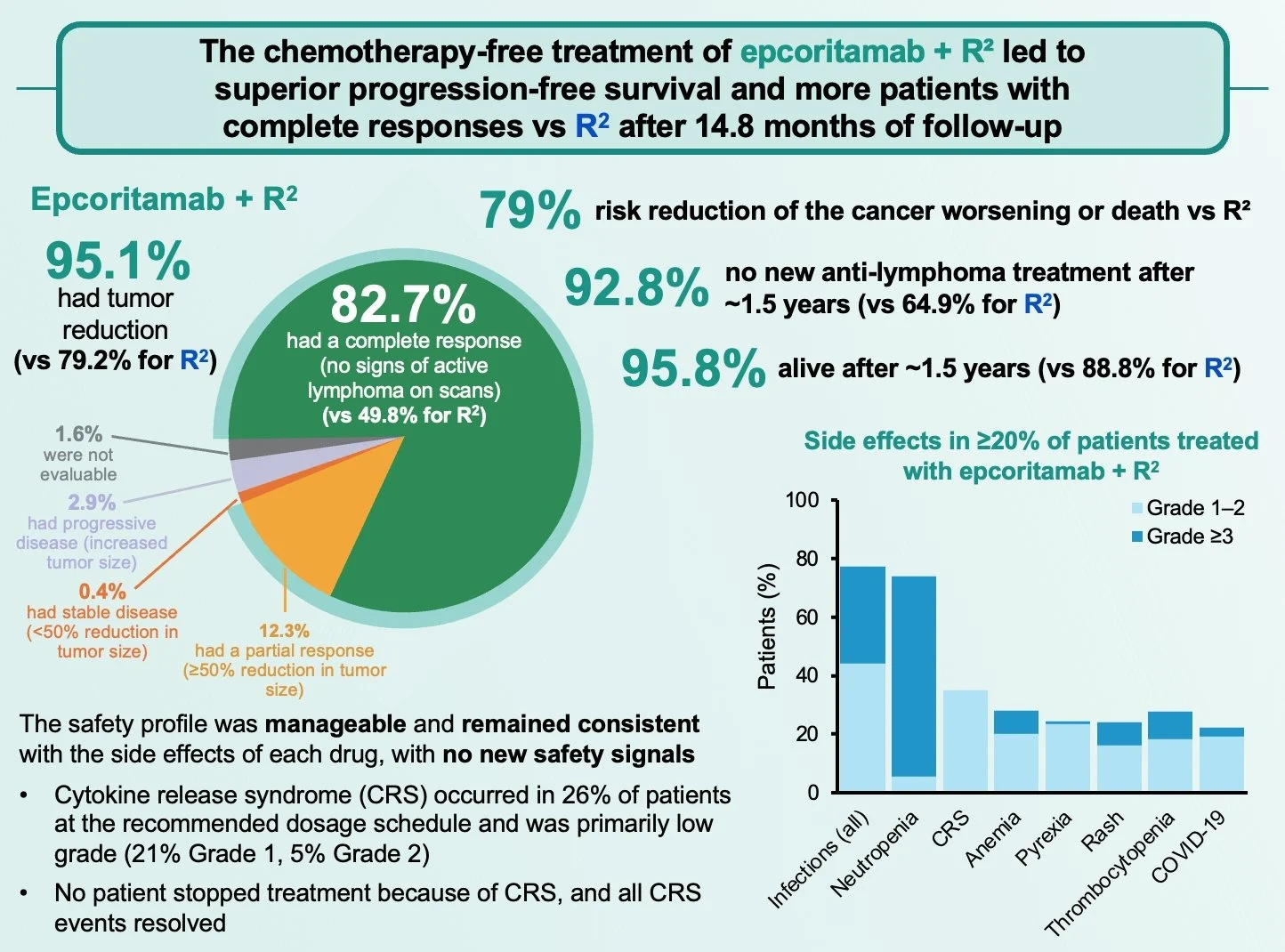
- ORR: 95.7% vs 81.0%
- CR: 83% vs 50%
- 79% reduction in risk of progression or death (HR 0.21)
Benefit consistent across prespecified subgroups.
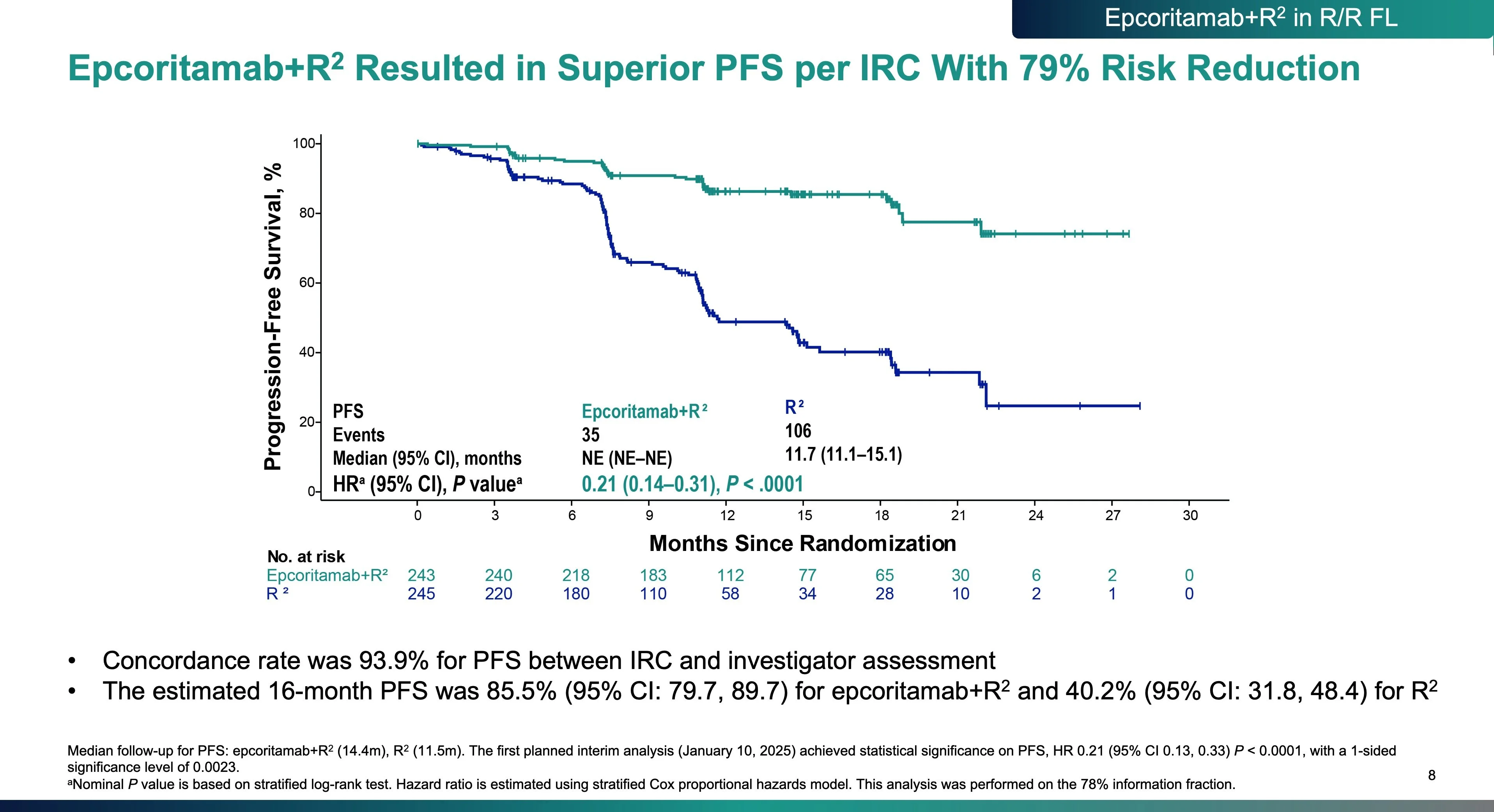
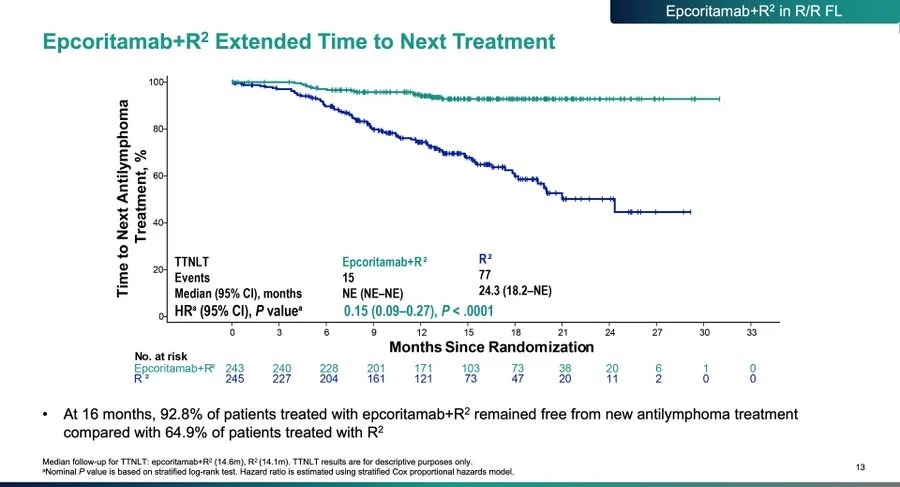
Beyond PFS, EPCORE FL-1 showed a meaningful improvement in time to next treatment, reinforcing the durability and real-world relevance of responses.
Despite still maturing follow-up, EPCORE FL-1 shows a statistically significant early OS signal, with separation of the curves and an HR of 0.38, favoring epcoritamab + R².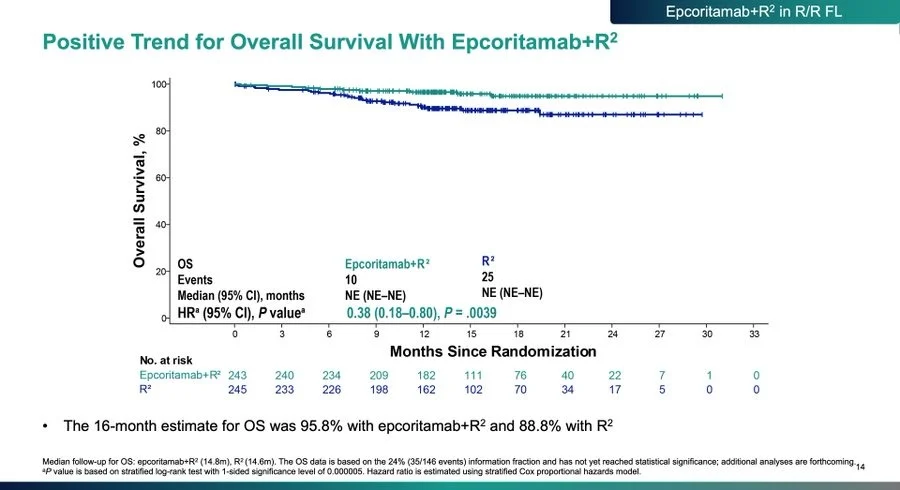
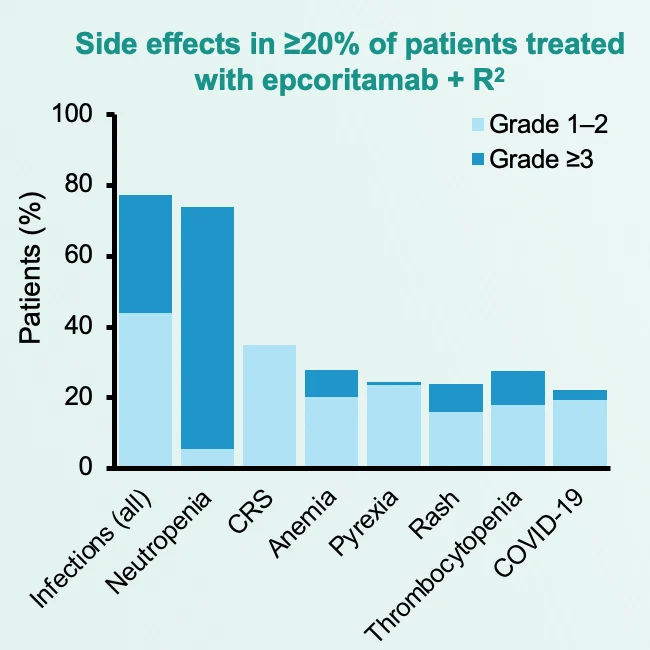
Safety profile was manageable and predictable, with no new safety signals:
- CRS mostly grade 1–2; no grade ≥3 CRS
- No tx discontinuations due to CRS
- Neutropenia and infections were manageable
- >90% relative dose intensity maintained Compatible with outpatient delivery.
Why this matters:
- First bispecific antibody approved in combination for FL
- Fixed-duration, chemotherapy-free regimen
- Immediate impact on treatment sequencing and clinical practice
Proud to represent Brazil in this milestone and to be part of a global study with strong participation from Brazilian centers, contributing to a change in standard of care.
Taken together, EPCORE FL-1 shows that epcoritamab + R² achieves deep and durable responses, delays the need for subsequent therapy, and maintains a predictable, manageable safety profile, supporting its role as a new standard of care.
For those who want to dive deeper, the full EPCORE FL-1 data are published in The Lancet and have already led to FDA and ANVISA approval.”


