
Top Colorectal Cancer Trials to Watch at ESMO 2025
The ESMO 2025 Congress is poised to deliver practice-shaping data in colorectal cancer, one of the most intensively studied and rapidly evolving areas of GI oncology. From first-line immunotherapy combinations to next-generation targeted agents and biomarker-driven treatment strategies, several highly anticipated trials are expected to make headlines.
This overview highlights the most clinically relevant colorectal cancer trials to be presented at ESMO 2025, with potential to inform practice and shape future standards of care.
Final results of the PEGASUS trial: Post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II colon cancer patients
Abstract: 723O
Presenter: Silvia Marsoni (Milan, Italy, (TO))
Trial Type: Phase II
Session: Proffered Paper session 1 – GI tumours, lower digestive
The PEGASUS trial explored whether liquid biopsy (ctDNA) could guide post-surgical and adjuvant management in stage II–III colon cancer. Retrospective studies had already shown ctDNA positivity as a strong predictor of relapse, but its role in tailoring therapy was unclear.
In this phase II study, presented at ESMO 2023, 135 patients across Italy and Spain underwent ctDNA testing after surgery using Guardant Reveal. ctDNA-positive patients received CAPOX, while ctDNA-negative patients received CAPE, with repeat testing to adjust therapy and reduce false negatives. At the end of treatment, ctDNA results guided escalation (to FOLFIRI) or de-escalation.
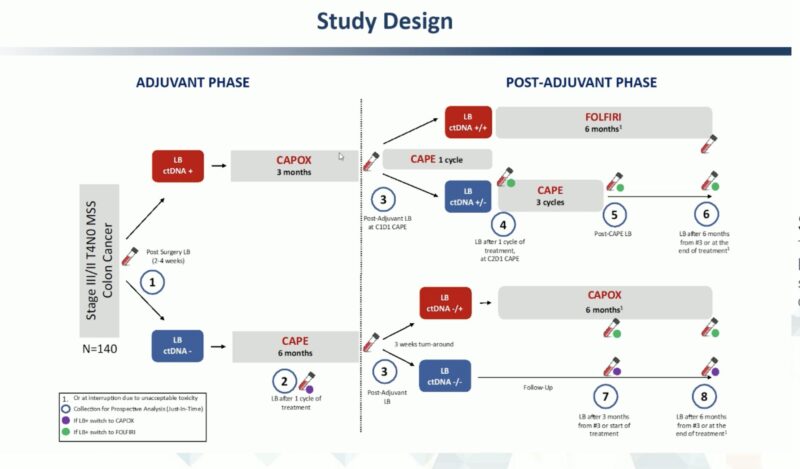
source: ESMO 2023 (27-07-2023)
Key Results
- Median follow-up: 20.8 months
- 26% of patients were ctDNA-positive, with significantly higher relapse risk (HR 4.37, p=0.0003)
- Switching ctDNA-positive patients to FOLFIRI converted ~50% to ctDNA-negative, many remaining relapse-free
- Only 9 ctDNA-negative patients relapsed
These results suggest liquid biopsy can refine adjuvant therapy, avoiding unnecessary toxicity while potentially improving outcomes.
FOxTROT and NICHE-2 trials: Comparison of outcomes in clinical trials of locally advanced dMMR colon cancer
Abstract:724O
Presenter: Jenny Seligmann (Leeds, United Kingdom, Yorkshire)
Trial Type: FOxTROT phase III, NICHE-2 phase II
Session: Proffered Paper session 1 – GI tumours, lower digestive
Both FOxTROT and NICHE-2 trials were tested in locally advanced colon cancer to explore pre-surgical treatment strategies. FOxTROT (phase III) focused on neoadjuvant chemotherapy, while NICHE-2 (phase II, results published in NEJM 2024) evaluated neoadjuvant immunotherapy with nivolumab plus ipilimumab in dMMR tumors.
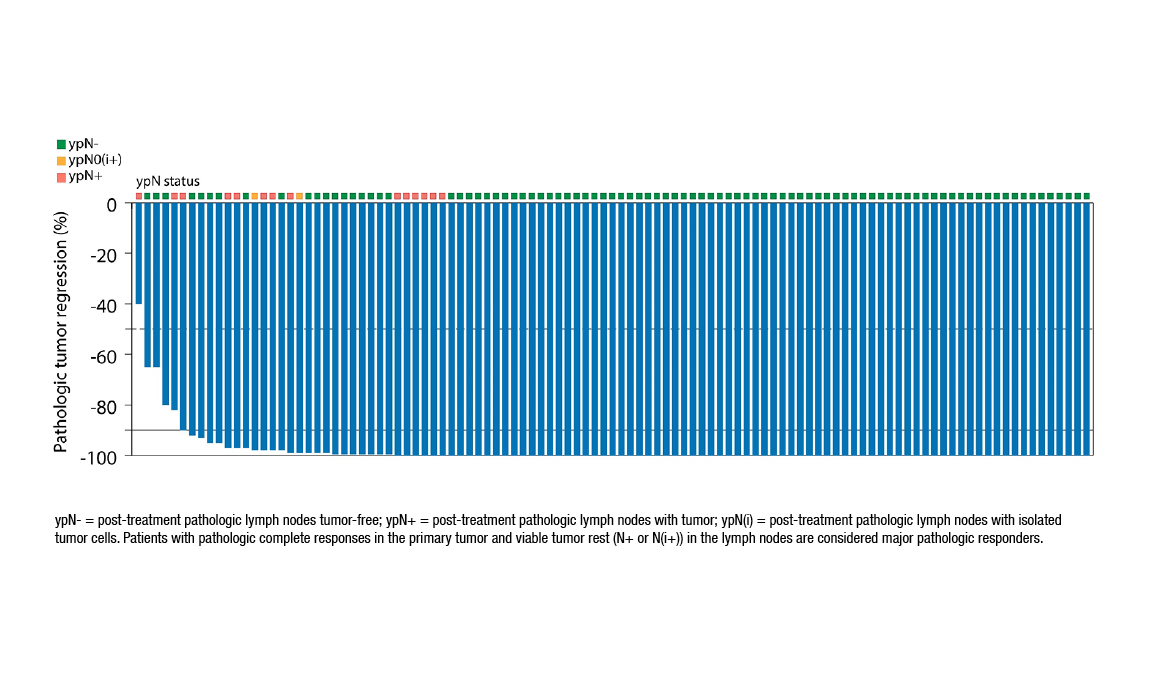
Source: Niche-2 trial Results ESMO Congress 2022
NICHE-2 achieved:
- 95% major pathological response
- 68% complete response
- No recurrences at 26 months
- Minimal severe toxicity
These trials will be presented together in a session comparing outcomes in locally advanced dMMR colon cancer, highlighting differences between chemotherapy and immunotherapy approaches.
Read about Opdivo (Nivolumab): Uses in Cancer, Side Effects, Dosages, Expectations on Oncodaily.
Translational analyses of AtezoTRIBE and AVETRIC trials: Leveraging Artificial Intelligence to predict ICI efficacy in proficient MMR mCRC
Abstract:725O
Presenter: Martina Carullo (Pisa, Italy)
Trial Type: phase II
Session: Proffered Paper session 1 – GI tumours, lower digestive
Both AtezoTRIBE and AVETRIC explored immune checkpoint inhibitors (ICIs) in metastatic colorectal cancer (mCRC). AtezoTRIBE, a Phase II trial, tested FOLFOXIRI plus bevacizumab with or without atezolizumab. Translational analyses showed that patients with high Immunoscore-IC or high tumor mutational burden (TMB) had the greatest benefit, highlighting potential predictive biomarkers.
AtezoTRIBE results, published in JCO 2024, reported 4-year outcomes in 218 patients:
- Median OS: 33.0 vs. 27.2 months (atezolizumab vs. control, HR 0.78), 30.8 vs. 29.2 months in the pMMR subgroup (HR 0.80)
- PFS benefit: 13.1 vs. 11.5 months (HR 0.72)
- Safety remained manageable with no new treatment-related deaths.
AVETRIC (NCT04513951) is a Phase II study evaluating avelumab with FOLFOXIRI and cetuximab in RAS/BRAF wild-type, MSS, pMMR mCRC patients.
Updated translational results from both trials will be presented, leveraging artificial intelligence to predict ICI efficacy in pMMR mCRC.
OPTIPRIME trial results: “Stop-and-Go” FOLFOX Plus Panitumumab in First-Line RAS/BRAF Wild-Type mCRC
Abstract: 727MO
Presenter: Jean-Baptiste Bachet (Paris, France)
Trial Type: phase II
Session: Mini oral session — GI tumours, lower digestive
The OPTIPRIME trial (FFCD 1605) tested first-line FOLFOX plus panitumumab in RAS wild-type mCRC using a “stop-and-go” approach.
- Six cycles of induction therapy
- Maintenance with fluoropyrimidine
- Reintroduction of FOLFOX + panitumumab upon progression
This strategy may maximize efficacy while minimizing toxicity, offering a more tolerable, personalized first-line approach for RAS/BRAF wild-type mCRC patients.
Anti-EGFR Rechallenge: Role of Low-Frequency Mutations and rMAFs in RAS/BRAF WT mCRC
Abstract: 728MO
Presenter: Pau Mascaró Baselga (Barcelona, Spain)
Trial Type: phase II
Session: Mini oral session — GI tumours, lower digestive
This study investigates how low-frequency resistance mutations and relative mutant allele frequencies (rMAFs) can predict response to anti-EGFR rechallenge in patients with RAS/BRAF wild-type metastatic colorectal cancer. The goal is to identify which patients are most likely to benefit from repeated EGFR-targeted therapy, guiding more personalized treatment decisions.
BREAKWATER Trial: ctDNA and Genomic Changes in BRAF V600E–Mutant mCRC Patients
Abstract: 729MO
Presenter: Scott Kopetz (Houston, United States of America)
Trial Type: phase III
Session: Mini oral session — GI tumours, lower digestive
The BREAKWATER trial (NCT04607421) is a global, open-label, phase 3 study evaluating first-line encorafenib plus cetuximab (EC), with or without FOLFOX chemotherapy, versus standard care in patients with untreated BRAF V600E–mutant metastatic colorectal cancer. Results published in JCO 2025 showed that EC+FOLFOX significantly improved objective response rates compared with standard chemotherapy.
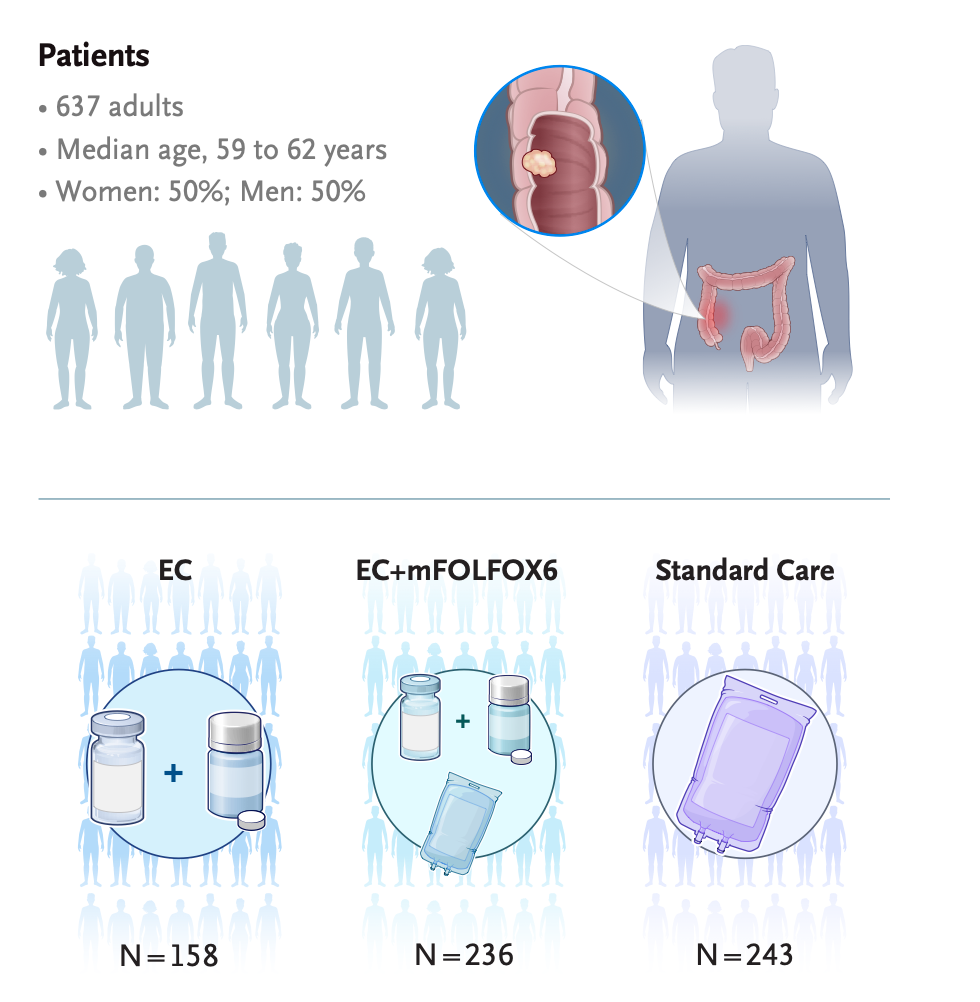
Source: Elez E et al. | 10.1056/NEJMoa2501912 | Published on May 30, 2025 NEJM
Key findings:
- Objective response rate: 60.9% vs 40.0% (OR 2.44; P=0.0008)
- Rapid, durable responses and promising OS
- Interim overall survival data also suggest a meaningful benefit.
- Safety was manageable and consistent with known profiles.
These findings support EC+FOLFOX as a highly active first-line option in this aggressive mCRC subtype. ctDNA analyses from BREAKWATER will be presented, tracking BRAF V600E dynamics and genomic changes during treatment to explore mechanisms of response and resistance.
TriComB study results: Trifluridine/Tipiracil + Capecitabine & Bevacizumab as First-Line Therapy in mCRC
Abstract: 730MO
Presenter: Veronica Conca (Pisa, Italy)
Trial Type: phase I/II
Session: Mini oral session — GI tumours, lower digestive
The TriComB trial is a Phase I/II multicenter study evaluating the safety and activity of trifluridine/tipiracil combined with capecitabine and bevacizumab in patients with metastatic colorectal cancer who are not eligible for intensive chemotherapy. Part 1 tested the optimal dose, while Part 2 assessed treatment activity and response at that dose. The regimen is given in 28-day cycles until disease progression, unacceptable toxicity, or patient choice.
The upcoming presentation will report the first phase II results, including response rates, safety, and early signals of efficacy for this upfront combination therapy.

Read more Lonsurf (trifluridine/tipiracil) 2025 Updates: Uses in Cancer, Side Effects, Dosage, Expectations on OncoDaily.
Phase 1 Dose Expansion Results: Telisotuzumab Adizutecan (ABBV-400; Temab-A) + Bevacizumab vs Standard of Care in 3L+ Colorectal Cancer
Abstract: 731MO
Presenter: Michael Cecchini (New Haven, United States of America)
Trial Type: phase I
Session: Mini oral session — GI tumours, lower digestive
Telisotuzumab adizutecan (ABBV-400; Temab-A) is an antibody-drug conjugate targeting c-Met, a receptor often overexpressed in colorectal cancer. In this Phase 1 dose-expansion study, Temab-A was combined with bevacizumab and compared to standard-of-care therapy in patients with 3L+ metastatic colorectal cancer.
The trial evaluates safety, tolerability, and preliminary activity, aiming to determine whether targeting c-Met alongside VEGF inhibition can provide clinical benefit in heavily pretreated CRC patients.
Takeaway
ESMO 2025 will highlight precision-guided, biomarker-driven, and innovative treatment strategies in colorectal cancer. From liquid biopsy-guided therapy to next-generation immunotherapy and targeted combinations, these trials collectively advance personalized care, improved outcomes, and optimized treatment selection in CRC.

Read more about Top 10 Immunotherapy Trials in Lung Cancer to Watch at ESMO 2025 on OncoDaily.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
