Lonsurf (trifluridine/tipiracil) is an oral antineoplastic agent composed of a nucleoside analog and a thymidine phosphorylase inhibitor, developed to enhance the cytotoxic activity of trifluridine through improved systemic exposure. Since its initial approval, Lonsurf has become a part of treatment strategies for patients with refractory metastatic cancers, particularly when standard options have been exhausted.
In recent years, ongoing studies and real-world data have continued to refine its clinical utility, safety profile, and potential for combination approaches. As evidence accumulates, understanding the updated role of Lonsurf in 2025 is crucial for optimizing patient outcomes. This article will explore its current and emerging indications, safety and tolerability, dosing considerations, and treatment expectations.
Which Company Produced Lonsurf?
Lonsurf is developed by Taiho Pharmaceutical Co., Ltd., a Japanese company with a strong focus on oncology. Taiho is part of the Otsuka Group and led the discovery and initial development of Lonsurf. To expand global access and development, Taiho entered into a strategic partnership with Servier, a French independent pharmaceutical company.
Under this collaboration, Taiho retained rights for Japan and parts of Asia, while Servier acquired exclusive rights for development and commercialization in other global markets, including the U.S., Europe, and Latin America. This partnership allowed for broader regulatory submissions, large-scale clinical trials, and worldwide distribution. The alliance combines Taiho’s oncology innovation with Servier’s extensive global infrastructure and commercialization experience.
In Asia, Taiho’s business partner TTY Biopharm launched Lonsurf in Taiwan (around mid‑2018) and Jeil Pharmaceutical is preparing its market launch in South Korea.
How Does Lonsurf Work?
Trifluridine/tipiracil is an oral antineoplastic agent composed of two active components: trifluridine, a thymidine-based nucleoside analog, and tipiracil hydrochloride, a thymidine phosphorylase inhibitor. The combination is designed to optimize the pharmacologic activity of trifluridine by enhancing its systemic exposure. Trifluridine is incorporated directly into DNA during the S-phase of the cell cycle, where it interferes with DNA synthesis and function, ultimately leading to tumor cell death. Its antitumor effect is primarily attributed to this incorporation into DNA rather than inhibition of thymidylate synthase, which distinguishes it from other fluoropyrimidines such as 5-FU.
Tipiracil, on the other hand, has minimal direct antitumor activity but plays a critical role in preventing the rapid degradation of trifluridine by inhibiting thymidine phosphorylase, a key enzyme responsible for trifluridine catabolism. By doing so, tipiracil increases the bioavailability and half-life of trifluridine, allowing for sustained cytotoxic concentrations in plasma. Together, the fixed-dose combination of trifluridine and tipiracil offers a synergistic mechanism that ensures effective DNA incorporation of trifluridine while overcoming its rapid metabolic clearance, thereby exerting cytotoxic effects in fluoropyrimidine-resistant tumors.
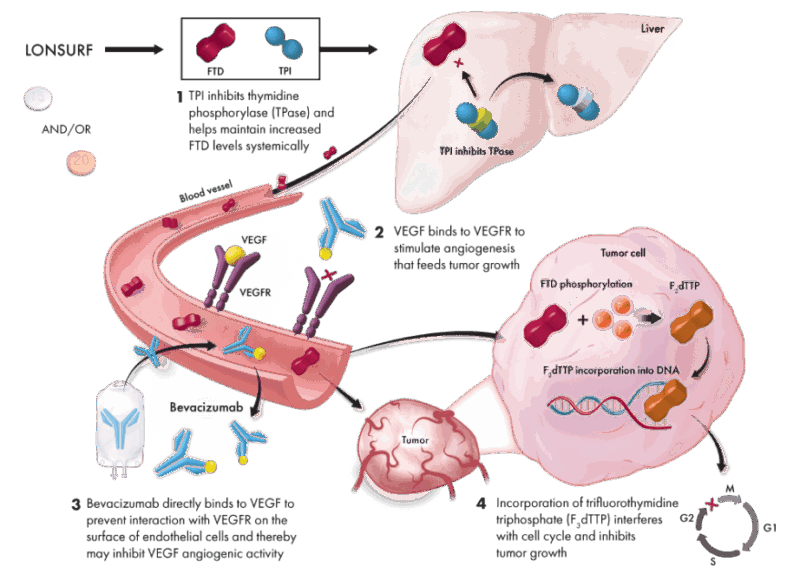
Source: Lonsurf Official Website
What Cancers Is Lonsurf Approved to Treat?
Trifluridine/tipiracil has received multiple FDA approvals for gastrointestinal cancers:
- In September 2015, Lonsurf was approved for metastatic colorectal cancer in patients who had progressed after standard therapies.
- In February 2019, it was approved for advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma after at least two prior chemotherapy regimens.
- In August 2023, the FDA expanded approval to include Lonsurf with bevacizumab for previously treated metastatic colorectal cancer, based on clinical trial data showing a significant survival benefit over Lonsurf alone.
These approvals support Lonsurf as a key option for patients with heavily pretreated gastrointestinal malignancies.
What research is behind the approval?
Trifluridine/tipiracil was approved based on data from multiple pivotal clinical trials across different gastrointestinal cancers. These studies evaluated its effectiveness and safety in patients who had progressed on standard therapies. Below, we’ll explore the key trials that supported its use in colorectal, gastric, and gastroesophageal junction cancers.
Lonsurf for mCRC
Lonsurf’s approval for metastatic colorectal cancer was supported by the phase III RECOURSE trial, published in the New England Journal of Medicine in 2015. This international, double-blind study enrolled 800 patients with refractory mCRC who had progressed after standard therapies. Patients were randomized 2:1 to receive Lonsurf (TAS-102) or placebo.
Trifluridine/tipiracil significantly improved overall survival: 7.1 months vs. 5.3 months with placebo (hazard ratio [HR] 0.68; 95% CI, 0.58–0.81; P<0.001). Progression-free survival was also longer (2.0 vs. 1.7 months; HR 0.48; P<0.001). Additionally, Lonsurf delayed deterioration in performance status (5.7 vs. 4.0 months; HR 0.66; P<0.001). The most common adverse events included neutropenia (38%), leukopenia (21%), anemia (18%), and febrile neutropenia (4%). One treatment-related death was reported. Overall, Lonsurf demonstrated a manageable safety profile and offered a clinically meaningful survival benefit in this heavily pretreated population.
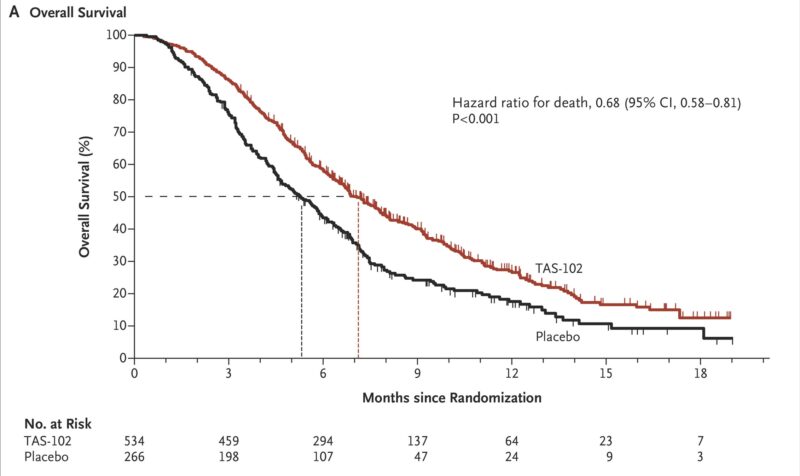
Lonsurf for Gastric/GEJ Cancer
Approval of Trifluridine/tipiracil for metastatic gastroesophageal junction cancer (mGEJC) is supported by a subgroup analysis from the global Phase III TAGS trial (NCT02500043), published in the Journal of Clinical Oncology in 2019. This trial evaluated the drug’s efficacy and safety in patients with advanced gastric or GEJ adenocarcinoma previously treated with at least two chemotherapy regimens.
Among the 507 patients enrolled, 145 had mGEJC as their sole primary disease site. These patients were randomized 2:1 to receive Lonsurf or placebo, both with best supportive care. In the mGEJC subgroup, median overall survival was 4.8 months with Lonsurf versus 3.5 months with placebo (HR 0.75), while progression-free survival was 1.9 months versus 1.8 months, respectively (HR 0.60).
The safety profile in mGEJC patients was consistent with the overall population. Grade ≥3 adverse events were more common with Lonsurf (77% vs. 59%), with neutropenia (25%) and anemia (13%) being the most frequent. Despite a higher rate of prior therapies in the Lonsurf group, the treatment demonstrated clinical benefit and manageable toxicity in this heavily pretreated patient population.

Learn more about Stomach Cancer: Symptoms and Causes, Types, Diagnosis and Treatment on OncoDaily.
Lonsurf + Bevacizumab for mCRC
The approval of Trifluridine/tipiracil in combination with bevacizumab for previously treated metastatic colorectal cancer is supported by results from the SUNLIGHT trial, published in The New England Journal of Medicine in May 2023.
This randomized phase 3 trial enrolled 492 patients with refractory metastatic colorectal cancer who had received up to two prior lines of systemic therapy. Patients were assigned to receive either Lonsurf with bevacizumab or trifluridine/tipiracil alone. The combination significantly improved overall survival: median OS was 10.8 months versus 7.5 months with Lonsurf monotherapy (hazard ratio [HR] 0.61; P<0.001). Progression-free survival was also improved (5.6 vs. 2.4 months; HR 0.44; P<0.001). Additionally, the time to ECOG performance status decline (from 0/1 to ≥2) was delayed with the combination (9.3 vs. 6.3 months; HR 0.54).
The safety profile was consistent with known effects of each agent. The most common side effects included neutropenia, nausea, and anemia, but no treatment-related deaths occurred. These findings highlight the clinical benefit of adding bevacizumab to Lonsurf in extending survival and preserving quality of life in this heavily pretreated population.
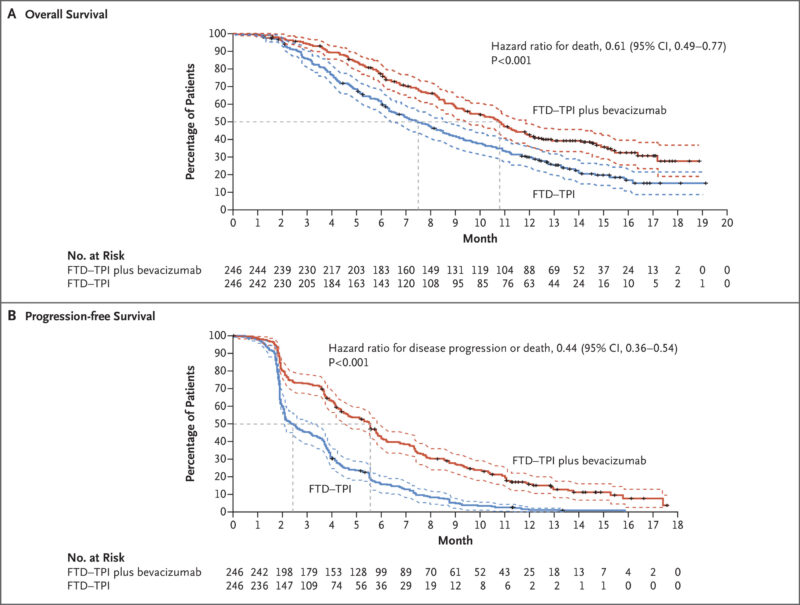
Lonsurf side effects and its management
Trifluridine/tipiracil is an oral chemotherapy used in the treatment of metastatic colorectal and gastric cancers. Like most anticancer drugs, it can cause side effects due to its effect on both cancer cells and healthy rapidly dividing cells, particularly those in the bone marrow and gastrointestinal tract. While many patients tolerate the medication well, side effects are common and vary in severity. Understanding and managing these symptoms early can help patients stay on treatment longer and maintain quality of life.
Common Side Effects
The most frequently reported side effect of Lonsurf is a decrease in white blood cell count, especially neutrophils, which can increase the risk of infections. Fatigue is also very common and may interfere with daily activities. Many patients experience anemia, leading to tiredness and shortness of breath, as well as thrombocytopenia, which may increase bruising or bleeding. Gastrointestinal issues such as nausea, vomiting, loss of appetite, and diarrhea are frequently reported. Abdominal discomfort, fever, and general weakness may also occur during treatment and usually follow a predictable pattern in each treatment cycle.
Less Common Side Effects
Some patients experience less common symptoms, including changes in taste, dry mouth, mouth sores, or mild skin reactions like rashes or itching. Hair thinning may be noticed but is typically not as prominent as with other chemotherapy drugs. Headaches and dizziness have also been observed in a smaller subset of patients. Although less frequent, these symptoms can still impact well-being and should be discussed with the treatment team.
Management of Side Effects
Management strategies focus on early identification and supportive care. Routine blood tests are performed before each treatment cycle to monitor white cells, red cells, and platelets. If neutropenia is significant, treatment may be delayed, the dose may be reduced, or the doctor may prescribe medications such as granulocyte-colony stimulating factors (G-CSF) to stimulate white cell recovery. Anemia is generally managed through rest and nutritional support, and in some cases, a transfusion may be necessary.
For nausea and vomiting, antiemetics are typically prescribed before and during treatment. Diarrhea may be managed with dietary changes and antidiarrheal medications such as loperamide. Fatigue often improves with rest, light physical activity, and proper hydration. For less common side effects like mouth sores or taste changes, gentle oral hygiene and supportive rinses can help. Skin reactions may be treated with topical creams or antihistamines if needed. Regular communication with the healthcare team is essential. Early reporting of symptoms allows timely interventions and helps maintain the balance between effective cancer control and tolerable treatment.

What is the Recommended Dosage of Lonsurf?
Trifluridine/tipiracil is available as oral tablets in two strengths: 15 mg/6.14 mg and 20 mg/8.19 mg. For metastatic colorectal cancer, it is approved as monotherapy or in combination with bevacizumab in patients previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based regimens, anti-VEGF therapy, and, if RAS wild-type, anti-EGFR agents. The recommended dose is 35 mg/m² (based on the trifluridine component) twice daily on Days 1–5 and 8–12 of each 28-day cycle, not exceeding 80 mg per dose. Bevacizumab (5 mg/kg IV) may be added on Days 1 and 15.
In advanced gastric or gastroesophageal junction cancer, trifluridine/tipiracil is used after at least two prior lines of therapy, with the same dosing schedule (35 mg/m² BID, Days 1–5 and 8–12 of each 28-day cycle). Adjust based on blood counts and toxicity. Withhold treatment for severe neutropenia, thrombocytopenia, or grade 3–4 side effects, then resume at a reduced dose. Up to three dose reductions allowed (minimum 20 mg/m² BID). No re-escalation permitted.
How is Lonsurf Administered?
Trifluridine/tipiracil is taken orally with food, based on the trifluridine dose rounded to the nearest 5 mg. Tablets must be swallowed whole. Missed doses should not be replaced. As a cytotoxic agent, it requires special handling. Store at 20–25°C; if removed from the original bottle, use within 30 days.
Lonsurf’s Metabolism and Elimination
Trifluridine and tipiracil are not metabolized through the cytochrome P450 system, minimizing drug-drug interaction risk. Trifluridine is primarily broken down by thymidine phosphorylase into an inactive metabolite, 5-(trifluoromethyl) uracil (FTY). At steady state, the half-life is approximately 2.1 hours for trifluridine and 2.4 hours for tipiracil.
What to Avoid During Lonsurf Treatment?
During treatment with Lonsurf, patients should take the medication exactly as prescribed—always with food and never making up for missed or skipped doses. The tablets must be swallowed whole and handled with care, as Lonsurf is a cytotoxic drug that requires special handling and disposal precautions. Patients should avoid starting any new medications, including over-the-counter drugs or supplements, without first consulting their healthcare provider. Although Lonsurf is not metabolized through the CYP enzyme system, drug interactions are still possible.
Pregnant women should not take Lonsurf, as it can harm the unborn baby. Effective contraception is essential during treatment and for at least six months afterward. Breastfeeding should also be avoided during therapy and for one day after the final dose. Throughout the course of treatment, it’s important to stay in close contact with the healthcare team and immediately report any signs of infection, unusual bleeding, or persistent fatigue.
Lonsurf effectiveness over time
A real-world analysis published in the Journal of Clinical Oncology (JCO, May 2025) evaluated treatment patterns and outcomes of trifluridine/tipiracil (Lonsurf) with or without bevacizumab in metastatic colorectal cancer (mCRC). Conducted across Texas Oncology practices between 2020 and 2024, the retrospective study included 265 patients, mostly receiving treatment in the third-line setting.
Patients treated with the combination of Lonsurf and bevacizumab had a median overall survival (OS) of 11.6 months, compared to 6.2 months with Lonsurf alone—showing a significant benefit (HR 2.1; p < 0.001). Time to next treatment or death was also longer with the combination (9.4 vs. 5.8 months). These findings align with the SUNLIGHT trial, supporting the added value of bevacizumab in this setting. The safety profile was similar between groups, though neutropenia was more frequent with the combination.
Ongoing trials with Lonsurf
The ongoing REGTAS-2 trial (NCT06992648), led by the Second Affiliated Hospital of Zhejiang University, is a randomized, multicenter phase II study evaluating whether trifluridine/tipiracil (Lonsurf) combined with regorafenib is non-inferior to Lonsurf plus bevacizumab in patients with refractory metastatic colorectal cancer (mCRC).
The trial aims to compare progression-free survival as the primary endpoint, along with overall survival, response rates, disease control, safety, and quality of life. Patients are randomized to receive either Lonsurf with regorafenib or with bevacizumab, continuing treatment until progression or unacceptable toxicity. Follow-up is conducted every 8 weeks until study completion or patient withdrawal.
Written by Mariam Khachatryan, MD
FAQ
What type of drug is Lonsurf?
Lonsurf (trifluridine/tipiracil) is an oral chemotherapy drug made up of two components: trifluridine, which damages cancer cell DNA, and tipiracil, which helps keep trifluridine active in the body for longer.
What cancers is Lonsurf approved to treat in 2025?
As of 2025, Lonsurf is FDA-approved for metastatic colorectal cancer and advanced gastric or gastroesophageal junction (GEJ) cancer.
What are the most common side effects of Lonsurf?
The most common side effects include low white blood cells (neutropenia), anemia, fatigue, nausea, vomiting, diarrhea, and loss of appetite. Regular blood tests are required to monitor safety during treatment.
How is Lonsurf taken?
Lonsurf is taken by mouth in tablet form, usually twice a day on specific days of a 28-day cycle (Days 1–5 and 8–12). It should always be taken with food, and the tablets must be swallowed whole.
Does Lonsurf cause hair loss?
Unlike many chemotherapy drugs, Lonsurf typically does not cause significant hair loss. Patients may notice mild hair thinning, but it is not as severe as with other chemotherapy agents.
How is Lonsurf metabolized and eliminated?
Lonsurf is not broken down by liver enzymes (CYP450). Trifluridine is mainly cleared by thymidine phosphorylase, with a half-life of about 2 hours.


