ESMO 2025 in Berlin is underway, bringing together leading researchers and clinicians from around the world. We reviewed the most promising immunotherapy trials in lung cancer, covering both small cell (SCLC) and non-small cell lung cancer (NSCLC), focusing on first-line therapies, targeted approaches, and novel immune modulators. Below is a curated list of the top 10 trials shaping the future of lung cancer treatment.
Tarlatamab with First-Line Chemoimmunotherapy for Extensive Stage Small Cell Lung Cancer (ES-SCLC): DeLLphi-303 Study
Presented by: Martin Wermke (Dresden, Germany)
Session: 2757O – Mini Oral session
Room: Bonn Auditorium
The DeLLphi-303 Phase 1b study investigates the safety and efficacy of tarlatamab in combination with carboplatin, etoposide, and PD-L1 inhibitors (atezolizumab or durvalumab) for first-line treatment of extensive-stage small cell lung cancer (ES-SCLC).
You Can Read More About Tarlatamab on OncoDaily
Mechanism of Action
Tarlatamab is a bispecific T-cell engager (TCE) that targets DLL3 on tumor cells and CD3 on T-cells. By binding to both, tarlatamab activates T-cells, directing them to kill DLL3-expressing cancer cells, enhancing immune-mediated tumor cell destruction. Combining this with PD-L1 inhibition aims to further enhance immune responses.
Why It Matters? This combination could offer a novel approach to improving outcomes in a cancer known for its aggressive nature and limited treatment options.
TeLuRide-005: Phase 2 Study of EIK1001, a Toll-Like Receptor 7/8 (TLR7/8) Co-Agonist with Pembrolizumab + Chemotherapy as First-Line Therapy in Stage 4 Non-Small Cell
Lung Cancer (NSCLC)
Presented by: Richard J. Gralla (Bronx, USA)
Session: 1850MO – Mini Oral session
Room: Berlin Auditorium – Hub 27
This Phase 2 study explores the combination of EIK1001, a TLR7/8 co-agonist, with pembrolizumab and chemotherapy (pemetrexed, carboplatin, or paclitaxel) for treating advanced stage 4 non-small cell lung cancer (NSCLC).
Mechanism of Action
EIK1001 activates Toll-like receptors 7 and 8, which stimulate a broad immune response, enhancing the ability of the immune system to recognize and destroy tumor cells. Combined with pembrolizumab, a PD-1 inhibitor, and chemotherapy, the goal is to boost T-cell activation and tumor targeting, overcoming immune resistance often seen in advanced NSCLC.
Why It Matters? This study offers an innovative strategy to improve immune activation in NSCLC, especially for patients with advanced disease who have limited treatment options.
Activity of Zipalertinib Against Active Central Nervous System (CNS) Metastases in Patients with Non-Small Cell Lung Cancer (NSCLC) Harboring EGFR Exon 20 Insertion (Ex20ins)/Other Uncommon Mutations
Presented by: Helena A. Yu (New York, USA)
Session: 1847MO – Mini Oral session
Room: Berlin Auditorium – Hub 27
This trial examines the efficacy of zipalertinib, a second-generation EGFR inhibitor, in treating EGFR Exon 20 insertion mutations in NSCLC patients with CNS metastases.
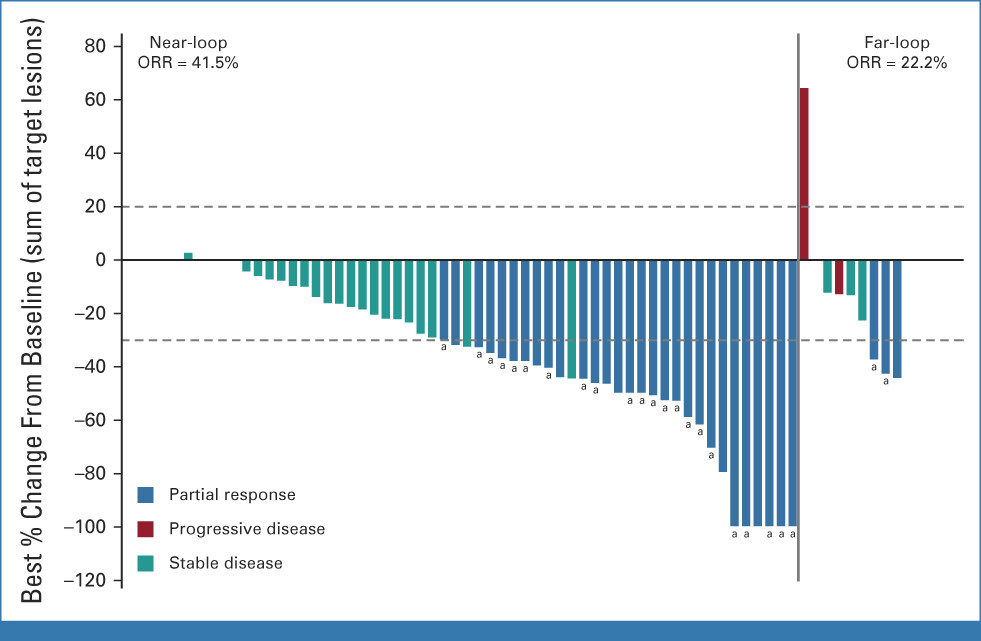
Figure: Tumor reduction and responses in the efficacy population by EGFR exon20ins location.
Source: Zofia Piotrowska et al. Safety, Tolerability, and Antitumor Activity of Zipalertinib Among Patients With Non–Small-Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Exon 20 Insertions. DOI:10.1200/JCO.23.00152
Mechanism of Action
Zipalertinib targets EGFR Exon 20 insertions, a mutation that drives cancer cell growth, offering more selective inhibition compared to earlier EGFR inhibitors that failed in this subset.
Why It Matters? Zipalertinib could provide a new, targeted treatment for NSCLC patients with EGFR Exon 20 insertions, especially those with CNS metastases, where treatments are limited.
Updated Results from a Phase 1/2 Study of Gocatamig for Small Cell Lung Cancer (SCLC) and Other Neuroendocrine Cancers
Presented by: Himisha Beltran (Boston, USA)
Session: 2758MO – Mini Oral session
Room: Hamburg Auditorium – CityCube A
This Phase 1/2 study investigates gocatamig (MK-6070), a novel immune modulator, for the treatment of relapsed/refractory SCLC and neuroendocrine cancers.
Mechanism of Action
Gocatamig is designed to enhance the immune system’s ability to recognize and destroy cancer cells. It works by activating the immune system, particularly T-cells, to target tumors more effectively. This is a promising approach for cancers like SCLC, where immune evasion is a major hurdle.
Why It Matters? This new immune modulator could provide a much-needed treatment option for aggressive and hard-to-treat cancers like SCLC, offering hope for patients with limited options.
DAREON-8: A Phase 1 Trial of First-Line Obrixtamig Plus Chemotherapy and Atezolizumab in Extensive-Stage Small Cell Lung Carcinoma (ES-SCLC)
Presented by: Solange Peters (Lausanne, Switzerland)
Session: 2759MO – Mini Oral session
Room: Hamburg Auditorium – CityCube A
This Phase 1 study investigates the combination of obrixtamig, chemotherapy (carboplatin + etoposide), and atezolizumab for first-line treatment of extensive-stage small cell lung carcinoma (ES-SCLC).
Mechanism of Action
Obrixtamig is a bispecific antibody that targets DLL3, a protein highly expressed in SCLC tumors, and CD3 on T-cells. By bridging T-cells and cancer cells, obrixtamig activates the immune system to kill DLL3-expressing tumor cells. Combined with chemotherapy and PD-L1 inhibition (atezolizumab), this combination aims to enhance immune-mediated tumor destruction.
Why It Matters: Obrixtamig could provide a novel therapeutic strategy for ES-SCLC, a particularly aggressive cancer, by leveraging immune activation alongside traditional therapies.
Intracranial Efficacy of Olomorasib, a Second-Generation KRAS G12C Inhibitor, in Patients with KRAS G12C-Mutant NSCLC Who Have Active, Untreated Brain Metastases
Presented by: Philippe Cassier (Lyon, France)
Session: 1846MO – Mini Oral session
Room: Berlin Auditorium – Hub 27
This study investigates the intracranial efficacy of olomorasib, a KRAS G12C inhibitor, in patients with KRAS G12C-mutant NSCLC and brain metastases.
Source: Lily official Website
Mechanism of Action
Olomorasib selectively targets the KRAS G12C mutation, which is present in some NSCLC tumors, preventing it from activating the downstream signaling pathways that promote tumor growth. This is crucial for patients with brain metastases, where current treatments often fall short.
Why It Matters? The results could provide a breakthrough treatment option for KRAS G12C-mutant NSCLC patients with brain metastases, a challenging subgroup to treat.
Updated Overall Survival Analysis from the Phase 2 PHAROS Study of Encorafenib Plus Binimetinib in Patients with BRAF V600E-Mutant Metastatic NSCLC
Presented by: Melissa L. Johnson (Nashville, USA)
Session: 1849MO – Mini Oral session
Room: Berlin Auditorium – Hub 27
This study presents updated overall survival data from the PHAROS Phase 2 trial evaluating the combination of encorafenib and binimetinib in BRAF V600E-mutant metastatic NSCLC.
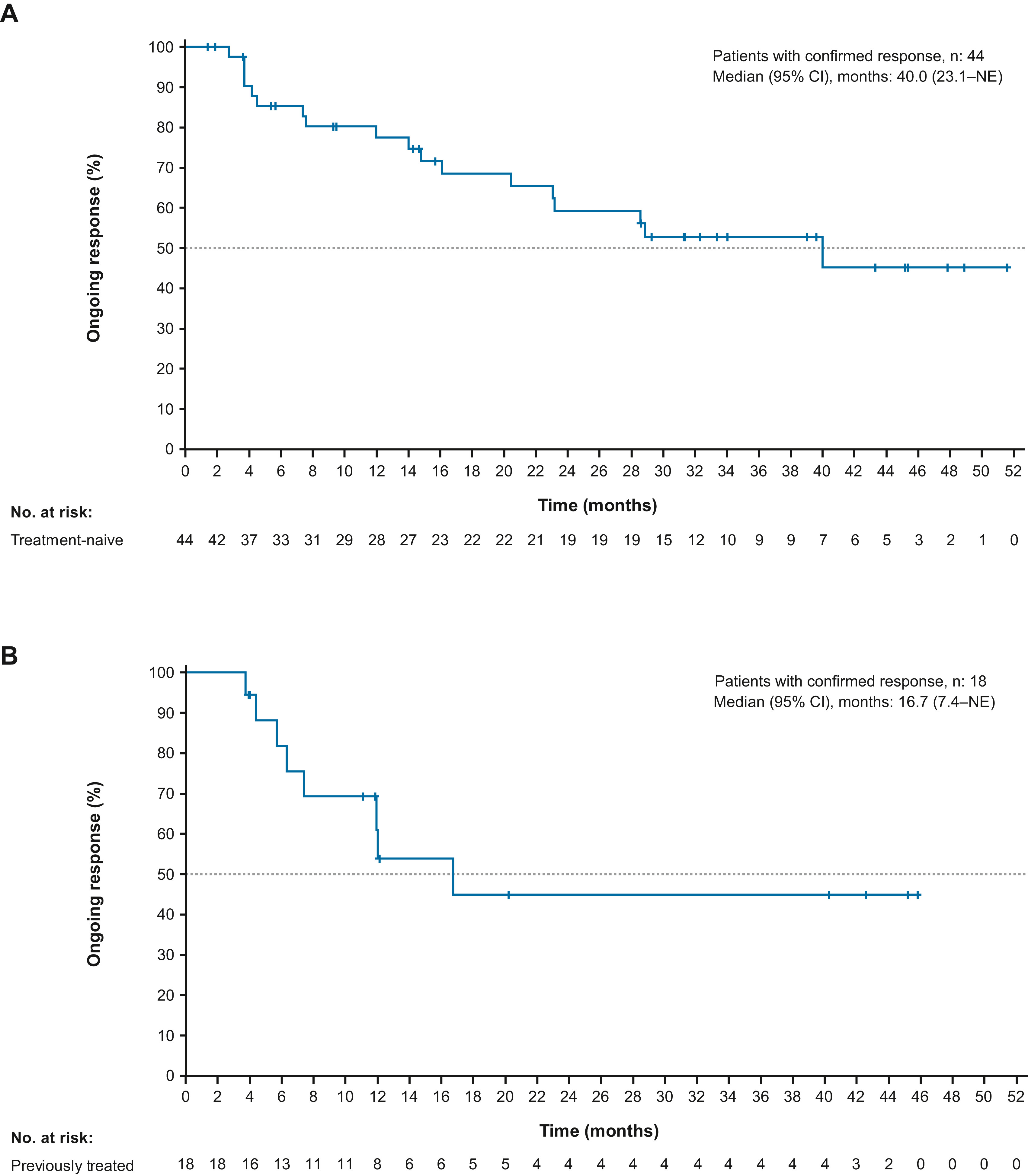
Figure From Updated Efficacy and Safety From the Phase 2 PHAROS study published in Journal of Thoracic Oncology: Kaplan-Meier estimate of DOR by IRR for (A) treatment-naive and (B) previously treated patients
Mechanism of Action
Encorafenib and binimetinib inhibit the BRAF and MEK pathways, respectively, which are frequently activated in BRAF V600E-mutant tumors, offering a targeted approach to treat this aggressive cancer.
Why It Matters? This trial could set a new standard for treating BRAF V600E-mutant metastatic NSCLC, a population that currently lacks effective therapies.
Pembrolizumab Plus Chemotherapy (PEM + CT) Versus Pembrolizumab (PEM) as First-Line Therapy for Advanced NSCLC with PD-L1 Tumor Proportion Score (TPS) ≥50%
Open-Label, Phase 3, Randomized Trial (PAULIEN)
Presented by: Ilias Houda (Amsterdam, Netherlands)
Session: 1851MO – Mini Oral session
Room: Berlin Auditorium – Hub 27
This Phase 3 trial compares pembrolizumab monotherapy versus pembrolizumab plus chemotherapy (pemetrexed or paclitaxel) for advanced NSCLC patients with high PD-L1 expression (TPS ≥50%).
Why It Matters? By directly comparing chemotherapy with immunotherapy, this study aims to determine the most effective first-line treatment for patients with high PD-L1 expression, refining treatment strategies for NSCLC patients.
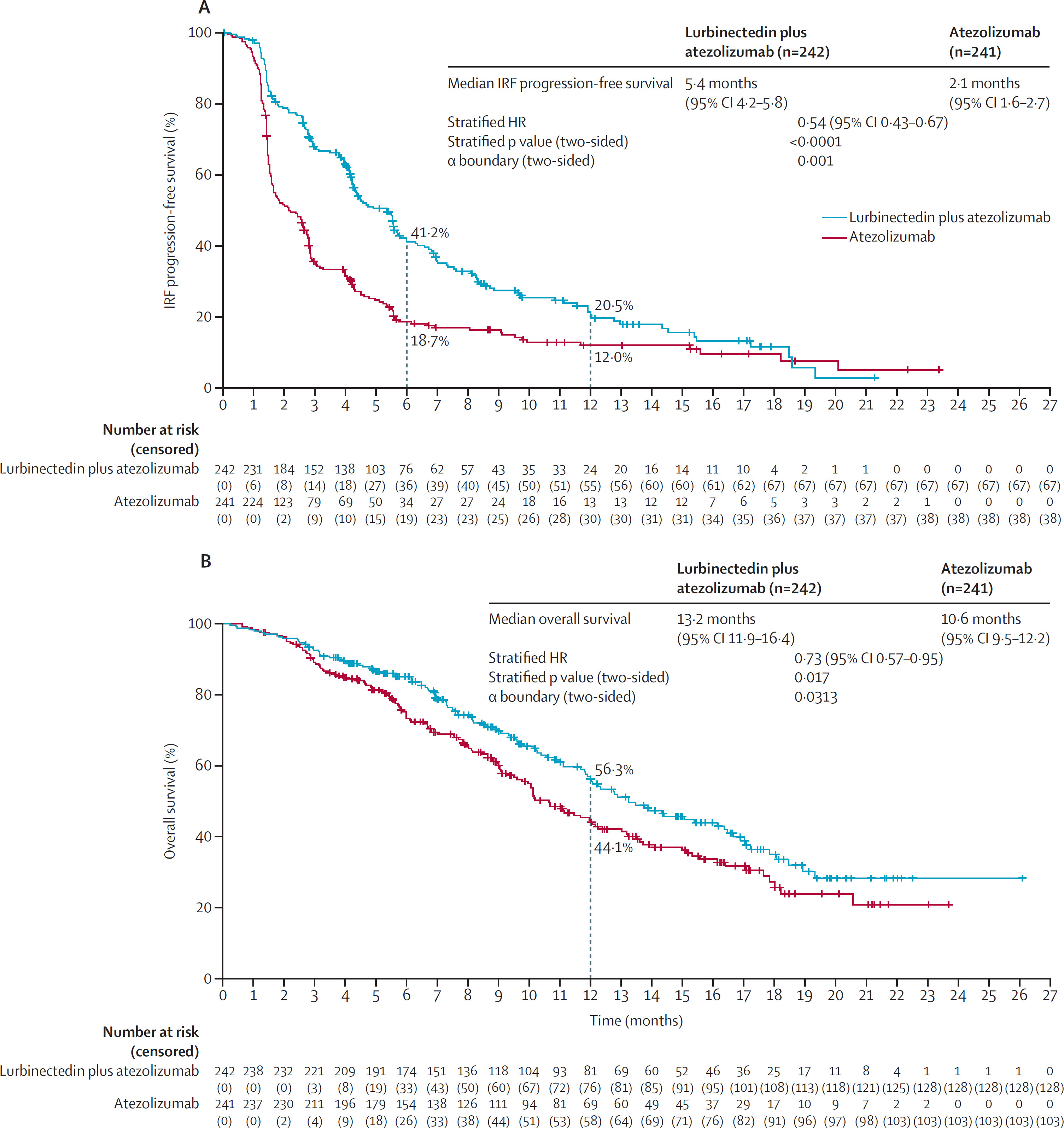
Efficacy and Safety of First-Line Maintenance Therapy with Lurbinectedin Plus Atezolizumab in Extensive-Stage Small-Cell Lung Cancer (IMforte): A Randomized, Multicenter, Open-Label, Phase 3 Trial
Presented by: Prof. Luis Paz-Ares, MD (Madrid, Spain)
Session: Hamburg Auditorium – CityCube A
Date & Time: TBC
This Phase 3 study compares lurbinectedin plus atezolizumab versus atezolizumab alone as maintenance therapy for treatment-naive ES-SCLC patients who do not experience progression after induction therapy with atezolizumab, carboplatin, and etoposide.
Figure From Original Article on IMForte trial Published in Lancet: IRF progression-free survival and overall survival from randomisation into the maintenance phase in the full analysis set
Mechanism of Action
Lurbinectedin inhibits RNA polymerase II, disrupting transcription and causing tumor cell death. Atezolizumab, a PD-L1 inhibitor, works by enhancing the immune system’s ability to attack and destroy cancer cells. The combination aims to improve progression-free survival (PFS) and overall survival (OS) in a patient group with poor prognosis.
Why It Matters? The IMforte study could change the standard of care for ES-SCLC maintenance therapy, providing an important new combination treatment for patients with this aggressive cancer.
A Phase 2, Multicenter, Randomized, Open-label Study of Ifinatamab Deruxtecan (I-DXd) in Subjects with Pretreated Extensive-Stage Small Cell Lung Cancer (ES-SCLC) (IDeate-Lung01)
Presented by: Pedro F. Simoes da Rocha (Barcelona, Spain)
Session: 2760MO – Mini Oral session
Room: Hamburg Auditorium – CityCube A
This Phase 2 study evaluates the efficacy, safety, and pharmacokinetics of ifinatamab deruxtecan (I-DXd) in patients with pretreatment-resistant ES-SCLC. The study has two parts: dose optimization (Part 1) and dose extension (Part 2), aiming to define the recommended Phase 2 dose of I-DXd and assess its anti-tumor activity in this population.
Mechanism of Action:
I-DXd is a B7-H3-targeting antibody-drug conjugate (ADC). It binds to B7-H3 on tumor cells and delivers a cytotoxic payload directly to cancer cells, promoting tumor cell death. I-DXd aims to improve outcomes in ES-SCLC by targeting B7-H3, a protein highly expressed in this cancer type.
Why It Matters? This study is crucial for optimizing the dose of I-DXd and could provide an effective treatment option for ES-SCLC, a cancer with limited treatment choices.
As we await the exciting data from these novel trials, updates will follow once the abstracts are released and presented onsite at ESMO 2025. Stay tuned to OncoDaily for live updates straight from the conference, where we’ll be bringing you the latest findings and in-depth coverage. Be sure to also check out OncoDaily’s GU Immuno-Oncology Trial Highlights, where we explore additional cutting-edge developments in the field. Keep an eye on our platform for timely insights and expert commentary from the event.