Capecitabine in Oncology: Mechanisms, Clinical Applications, Regimens, and Toxicity Management
Capecitabine is an orally administered fluoropyrimidine carbamate that serves as a prodrug of 5-fluorouracil (5-FU), a cornerstone agent in the treatment of solid tumors. Designed to mimic the pharmacokinetics of continuous 5-FU infusion, capecitabine offers the advantage of oral dosing and selective activation within tumor tissues. This tumor-specific conversion is mediated by the enzyme thymidine phosphorylase, which is often expressed at higher levels in malignant cells compared to normal tissues, enhancing therapeutic efficacy while minimizing systemic toxicity.
Since its approval, capecitabine has become a widely utilized agent in multiple cancer types, including colorectal, breast, gastric, and other gastrointestinal malignancies. Its use spans from monotherapy in early-stage settings to combination regimens in metastatic disease.
This article aims to provide a comprehensive overview of capecitabine’s role in modern oncology, covering its pharmacological mechanisms, approved clinical applications, dosing strategies, combination regimens, toxicity profile, resistance mechanisms, and emerging directions in research and precision medicine.
Mechanism of Action and Pharmacokinetics
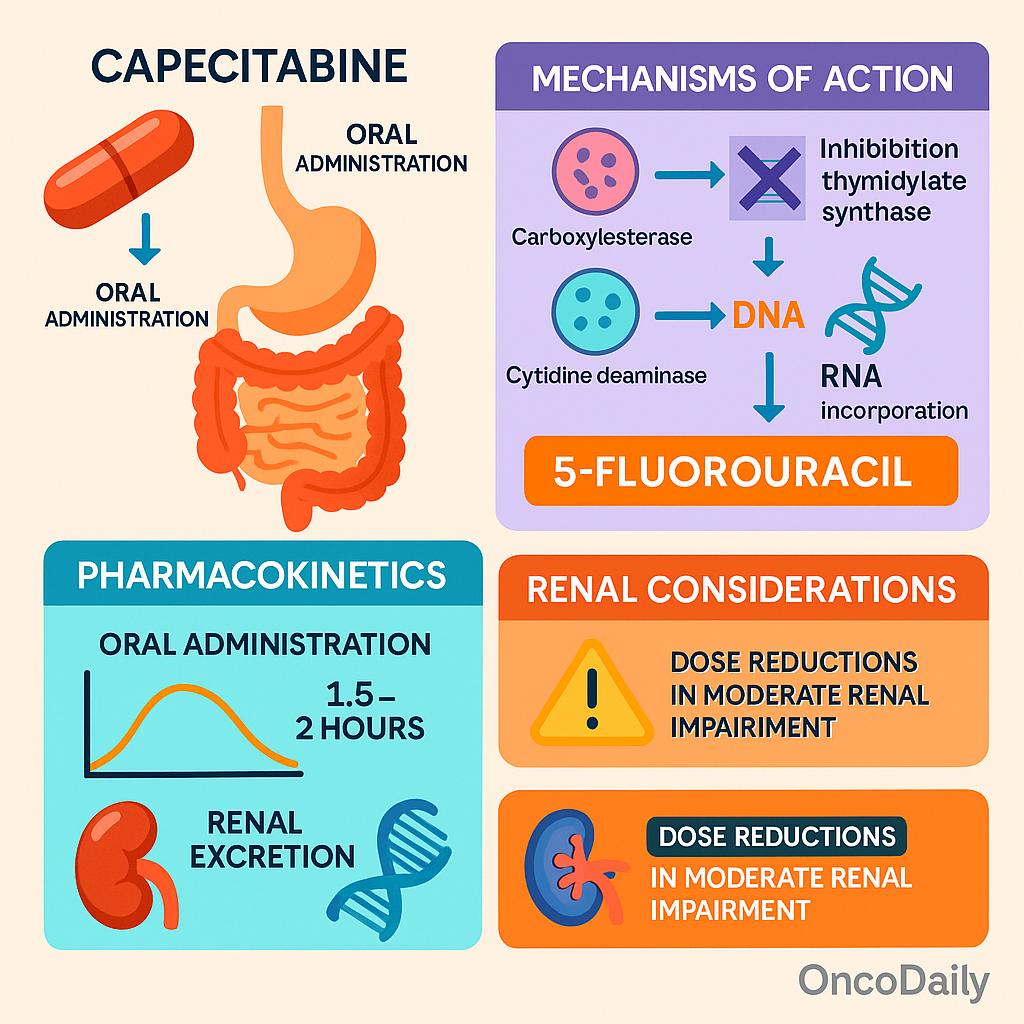
Capecitabine is pharmacologically inactive upon administration and must undergo a three-step enzymatic conversion to become cytotoxic. After oral absorption, capecitabine is first metabolized in the liver by carboxylesterase to 5‘-deoxy-5-fluorocytidine (5′-DFCR). This intermediate is then converted by cytidine deaminase, also primarily in the liver and peripheral tissues, to 5′-deoxy-5-fluorouridine (5′-DFUR). The final and crucial activation step occurs predominantly within tumor tissues, where thymidine phosphorylase—often overexpressed in malignant cells—converts 5′-DFUR into the active compound 5-fluorouracil (5-FU). This tumor-selective activation enhances antitumor activity while limiting systemic toxicity.
The cytotoxicity of 5-FU stems from two primary mechanisms. First, it inhibits thymidylate synthase (TS), an enzyme critical for the synthesis of thymidine triphosphate, a nucleotide essential for DNA replication and repair. TS inhibition leads to DNA damage and cell death, particularly in rapidly dividing cells. Second, 5-FU incorporates into RNA, disrupting normal RNA processing and function, which contributes to cytotoxicity in both malignant and normal proliferating tissues.
From a pharmacokinetic perspective, capecitabine is rapidly absorbed after oral administration, with peak plasma levels typically achieved within 1.5 to 2 hours. Its metabolism is primarily hepatic until the final activation step in tumor tissues. The drug and its metabolites are predominantly excreted through the kidneys. Therefore, renal function significantly influences capecitabine’s clearance and must be considered when adjusting doses. In patients with moderate renal impairment (creatinine clearance 30–50 mL/min), dose reductions are typically required, and the drug is contraindicated in those with severe renal dysfunction (CrCl <30 mL/min), as recommended in the NCCN guidelines.
Approved Clinical Indications
Capecitabine holds a well-established position in the treatment of several solid tumors, with multiple FDA-approved and NCCN-endorsed indications. According to the NCCN Colon Cancer Guidelines Version 2.2025, capecitabine is approved as adjuvant monotherapy in stage II and III colon cancer, particularly for patients who are not candidates for combination therapy. It is also widely used in combination with oxaliplatin (CAPOX regimen), which is considered equivalent to FOLFOX in efficacy for adjuvant and metastatic settings. CAPOX is particularly advantageous in scenarios favoring oral over infusion-based chemotherapy.
In breast cancer, capecitabine is FDA-approved for metastatic disease, especially in patients who have progressed on anthracyclines and taxanes. The NCCN Breast Cancer Guidelines Version 2.2025 list capecitabine as a recommended agent in hormone receptor-positive, HER2-negative metastatic breast cancer, often after endocrine resistance, and as part of combination strategies in triple-negative breast cancer. It also plays a role in post-neoadjuvant settings for patients with residual disease, as supported by the CREATE-X trial findings.
Capecitabine is additionally endorsed in gastric and gastroesophageal junction cancers, typically in combination regimens such as EOX (epirubicin, oxaliplatin, and capecitabine) or XELOX (capecitabine and oxaliplatin). These are included in the NCCN Gastric Cancer Guidelines Version 1.2025 as standard perioperative and first-line palliative treatments. Although not formally FDA-approved for pancreatic cancer, capecitabine is used off-label, particularly in combination with radiation or other agents (e.g., gemcitabine), in locally advanced or borderline resectable cases. It is also under investigation in clinical trials for enhancing radiosensitization or in maintenance strategies.
Capecitabine-Based Combination Regimens
Capecitabine serves as a cornerstone in several major chemotherapy regimens across gastrointestinal and breast cancers due to its oral administration and tumor-selective activation. One of the most widely used combinations is CAPOX (also known as XELOX), which pairs capecitabine with oxaliplatin. This regimen is considered a non-inferior alternative to the traditional FOLFOX regimen (5-FU, leucovorin, and oxaliplatin) in colorectal cancer. The XELOXA trial (NO16968)was a pivotal phase III study that compared CAPOX to bolus 5-FU/leucovorin in the adjuvant setting for colon cancer. It demonstrated that CAPOX was not only effective but also associated with improved disease-free survival in certain subgroups, establishing it as a standard option in adjuvant therapy, particularly for stage III disease.
In the treatment of upper gastrointestinal cancers, particularly advanced gastric and gastroesophageal junction cancers, the EOX regimen—a combination of epirubicin, oxaliplatin, and capecitabine—has emerged as a potent first-line option. The REAL-2 trial compared EOX with traditional ECF (epirubicin, cisplatin, and 5-FU) and demonstrated that EOX was not only non-inferior but also associated with improved median overall survival (11.2 months vs. 9.9 months), along with greater convenience due to oral capecitabine replacing infusional 5-FU. These findings supported EOX as a preferred regimen in the palliative setting.
Capecitabine is also incorporated in CapeCis (capecitabine plus cisplatin) and CapeOx regimens, both of which are used in the neoadjuvant or adjuvant treatment of rectal cancer, and increasingly in clinical trials for pancreatic cancer. In locally advanced rectal cancer, capecitabine is often used concurrently with radiation in total neoadjuvant therapy (TNT) approaches. Its oral route offers patients more autonomy and less need for central venous access, which is a major convenience advantage over continuous infusion 5-FU.
When comparing capecitabine-based regimens to those using infusional 5-FU, such as FOLFOX, data consistently suggest similar efficacy in terms of progression-free and overall survival across multiple trials and tumor types. However, capecitabine regimens offer logistical advantages: fewer clinic visits, no need for infusion pumps or ports, and better patient quality of life, albeit with a distinct toxicity profile including hand-foot syndrome and diarrhea.
Dosing and Administration
Capecitabine is typically dosed at 1250 mg/m² orally twice daily for 14 days, followed by a 7-day rest, in a 21-day cycle. When combined with radiation therapy, the dose is usually reduced to 825 mg/m² twice daily on radiation days to reduce toxicity.
Dose adjustments are important for renal impairment—patients with CrCl 30–50 mL/min should receive a reduced dose, and the drug is contraindicated if CrCl <30 mL/min. The elderly may also need lower starting doses due to increased risk of side effects. In cases of severe gastrointestinal or hematologic toxicity, treatment is typically paused and restarted at a reduced dose. NCCN guidelines (e.g., Colon Cancer v2.2025) recommend adjusting the dose based on patient tolerance, renal function, and toxicity severity.
Toxicity Profile and Side Effects
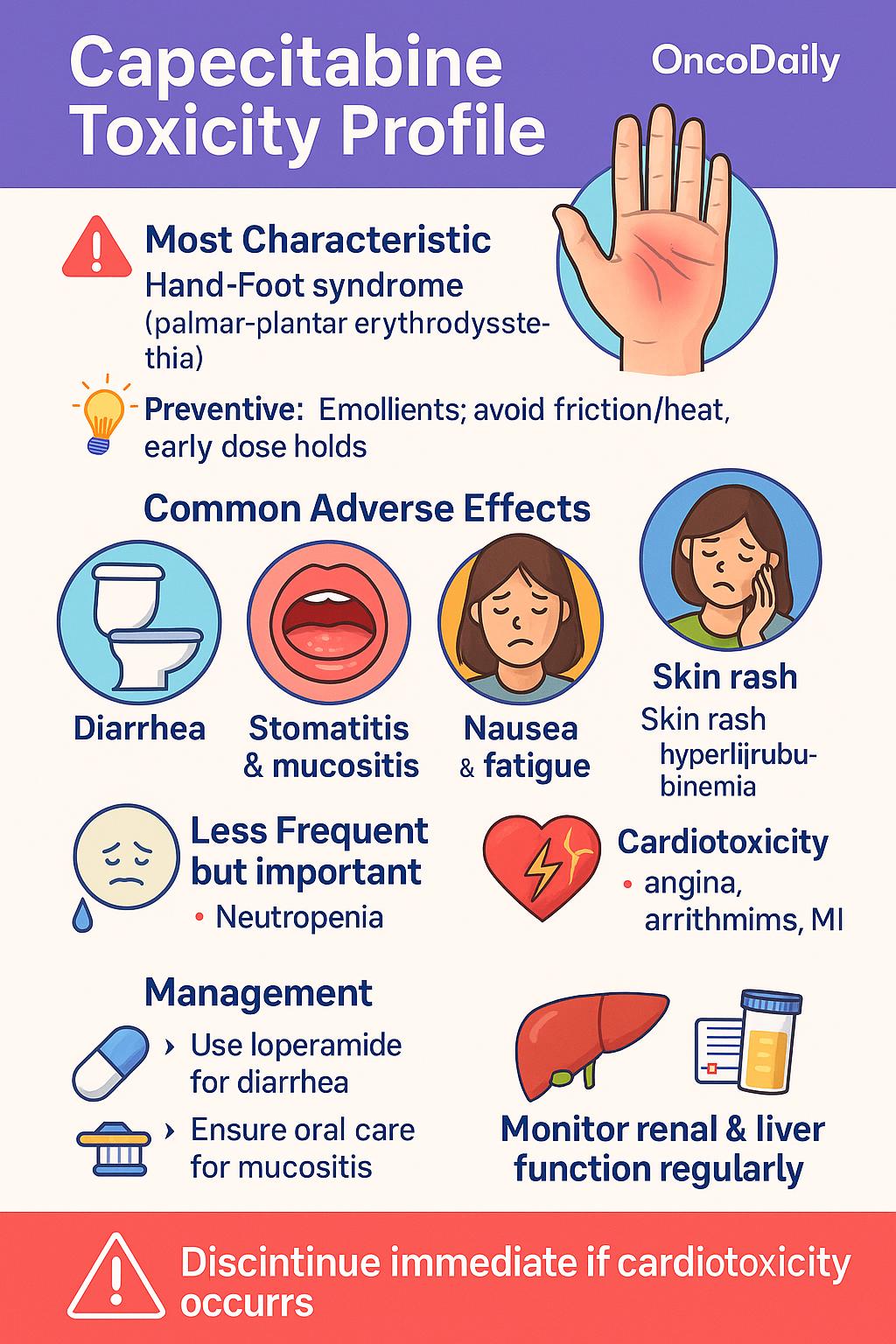
Capecitabine is generally well tolerated, but it has a well-defined toxicity profile that requires active monitoring. The most characteristic adverse effect is hand-foot syndrome (palmar-plantar erythrodysesthesia), which manifests as redness, swelling, numbness, and pain in the palms and soles. This can progress to blistering and functional impairment if not promptly managed. Preventive strategies include using emollients, avoiding friction or heat exposure, and dose interruptions at early signs.
Other frequent side effects include diarrhea, stomatitis, nausea, fatigue, and skin rash. These are typically mild to moderate but can become severe without appropriate supportive care. Antidiarrheal agents such as loperamide are used at the onset of symptoms, and oral hygiene measures can reduce mucositis and stomatitis risk.
Hematologic toxicity, particularly neutropenia, is less common compared to continuous infusion 5-FU, making capecitabine a convenient alternative in appropriate patients. However, elderly patients or those with renal impairmentare more prone to myelosuppression and gastrointestinal toxicity, necessitating dose adjustments. Rare but serious complications include cardiotoxicity (e.g., angina, arrhythmias, or myocardial infarction) and hyperbilirubinemia due to liver dysfunction. These events warrant immediate drug discontinuation and further evaluation.
Resistance Mechanisms and Biomarkers
Resistance to capecitabine, like other fluoropyrimidine-based therapies, is a significant clinical challenge that limits long-term efficacy in several cancers. Multiple mechanisms at the molecular level contribute to either intrinsic or acquired resistance:
- Upregulation of Thymidylate Synthase (TS): One of the primary mechanisms involves increased expression of thymidylate synthase, the target enzyme of 5-FU. Overexpression of TS allows tumor cells to maintain DNA synthesis even in the presence of the drug, leading to treatment failure. Several studies, including immunohistochemical analyses, have shown that higher TS levels correlate with poorer response to fluoropyrimidines, although this has not yet been incorporated into routine clinical decision-making.
- Reduced Activation via Thymidine Phosphorylase (TP): Capecitabine requires activation by thymidine phosphorylase (TP), which is more abundant in tumor tissue than in normal tissue. Decreased expression of TP limits conversion of capecitabine to its active form (5-FU) at the tumor site, resulting in reduced cytotoxic effect. This has been observed particularly in tumors with low angiogenic activity, as TP is often co-expressed with VEGF.
- Alterations in Apoptotic Pathways: Resistance can also emerge from changes in cellular apoptosis mechanisms. Tumors may exhibit reduced expression of pro-apoptotic proteins (like Bax) or increased levels of anti-apoptotic proteins (such as Bcl-2), allowing cells to evade programmed death despite DNA or RNA damage caused by 5-FU.
- Pharmacogenetic Variants and Predictive Biomarkers: Although efforts have been made to identify biomarkers predictive of capecitabine sensitivity—such as TS gene polymorphisms, DPD gene (DPYD) variants, and TP expression—none have yet reached universal clinical implementation. For instance, DPYD polymorphisms are known to predict toxicity but not efficacy, while TS expression assays suffer from variability in methodology and interpretation.
DPD Deficiency and Genetic Screening
Dihydropyrimidine dehydrogenase (DPD) is the principal enzyme responsible for the catabolism of fluoropyrimidines, including 5-fluorouracil (5-FU) and its oral prodrug, capecitabine. Over 80% of administered 5-FU is inactivated by DPD in the liver. When DPD activity is significantly reduced—either partially or completely—patients are at high risk for severe and potentially fatal toxicities, including neutropenia, mucositis, diarrhea, neurotoxicity, and hand-foot syndrome.
This enzyme is encoded by the DPYD gene, and inherited variants in this gene can result in decreased or absent DPD function. Complete deficiency is rare, but partial deficiency is more common and clinically significant.
As a result, DPYD genotyping or phenotyping is increasingly recommended prior to initiating capecitabine or 5-FU therapy, particularly in Europe. The European Medicines Agency (EMA) and several European oncology societies now advise pre-treatment DPYD testing to identify individuals at risk and guide initial dose reduction or alternative therapy. Common actionable DPYD variants include DPYD *2A, *13, c.2846A>T, and HapB3.
You Can Watch More on OncoDaily Youtube TV
Written by Toma Oganezova, MD
FAQ
What is capecitabine and how does it work?
Capecitabine (Xeloda) is an oral chemotherapy that converts into 5-fluorouracil (5-FU) in tumors, where it blocks DNA synthesis and damages cancer cells.
What cancers is capecitabine used for?
Capecitabine is commonly used for colorectal, breast, gastric, and rectal cancers, either alone or in combination regimens like CAPOX and EOX.
What are the most common side effects of capecitabine?
Common side effects include hand-foot syndrome, diarrhea, nausea, fatigue, and mouth sores. Severe effects can include cardiotoxicity or liver issues.
How is capecitabine dosed and taken?
Typical dose is 1250 mg/m² twice daily for 14 days followed by a 7-day break. Dose changes may be needed based on renal function or side effects.
What is hand-foot syndrome caused by capecitabine?
It’s a skin reaction causing redness, pain, or peeling on hands and feet. It’s a hallmark side effect of capecitabine and needs early management.
Should I get tested for DPD deficiency before starting capecitabine?
Yes, DPYD genotyping is recommended in some countries to prevent life-threatening toxicity in patients with DPD enzyme deficiency.
Can capecitabine be used with radiation therapy?
Yes, it’s often combined with radiation, especially in rectal cancer, using lower doses to enhance effectiveness while minimizing toxicity.
Is capecitabine better than 5-FU infusion?
It’s equally effective in many cancers and more convenient due to oral dosing, though it has a distinct toxicity profile.
Is capecitabine safe for patients with kidney problems?
Patients with moderate kidney impairment need dose adjustment. It’s not recommended if CrCl <30 mL/min.
Can older adults use capecitabine?
Yes, but they may be more sensitive to side effects. Lower starting doses are often used for elderly patients.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
