Pheochromocytoma: Causes, Diagnosis, Treatment, and Breakthrough 2025 Advances in Management
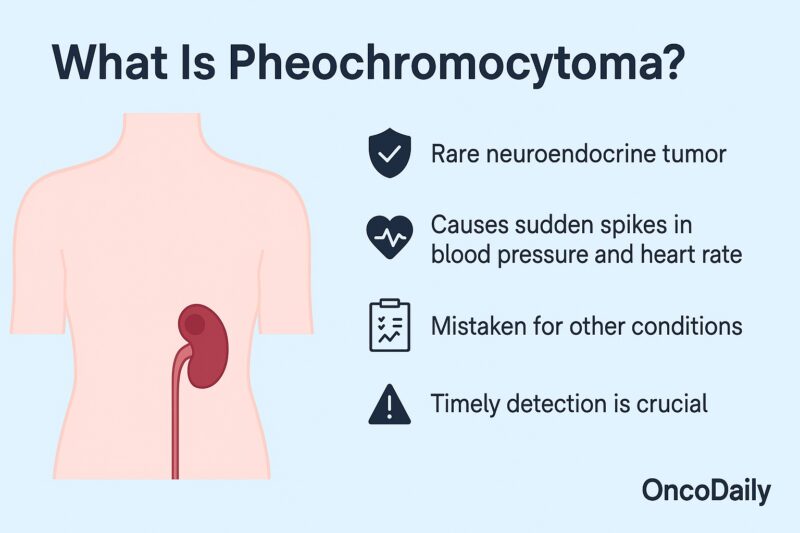
Pheochromocytoma is a rare adrenal tumor known for causing sudden spikes in blood pressure and heart rate. Often mistaken for other conditions, this tumor demands timely detection to avoid severe health complications. In this article, you’ll learn what pheochromocytoma is, how it’s diagnosed and treated, and why early recognition can significantly improve outcomes.
What Is Pheochromocytoma?
Pheochromocytoma is a rare neuroendocrine tumor that arises from the chromaffin cells in the adrenal medulla—the inner region of the adrenal glands, which sit atop the kidneys. These tumors secrete excess catecholamines, such as epinephrine (adrenaline) and norepinephrine (noradrenaline), leading to episodic or sustained hypertension and sympathetic overactivity.
Although pheochromocytomas are rare—occurring in about 2–8 per million people per year—they are critically important to recognize due to the potential for cardiovascular crises if left untreated. Approximately 85–90% of cases are benign, but 10–15% can be malignant, with metastases most often to lymph nodes, liver, lungs, or bones (Lenders et al., The Lancet, 2005).
Is Pheochromocytoma Cancerous? Benign vs Malignant Forms
Most pheochromocytomas are benign, meaning they do not invade surrounding tissues or spread to other parts of the body. However, about 10–15% of cases are malignant, which means the tumor has metastasized to sites such as the bones, lungs, liver, or lymph nodes (Lenders et al., The Lancet, 2005; Fishbein et al., J Clin Oncol, 2017).
It’s important to note that there are no definitive microscopic (histological) features that reliably distinguish benign from malignant pheochromocytoma; malignancy is diagnosed only when metastases are found in tissues where chromaffin cells are not normally present (Lenders et al., J Clin Endocrinol Metab, 2014).
Malignant pheochromocytomas are more likely in patients with certain genetic mutations (such as SDHB), larger tumor size, or extra-adrenal (paraganglioma) location (Fishbein et al., J Clin Oncol, 2017). Despite this, even benign pheochromocytomas can cause life-threatening complications if left untreated, due to their hormonal effects. Lifelong monitoring is therefore important for all patients following diagnosis and treatment.
Classification of Pheochromocytoma
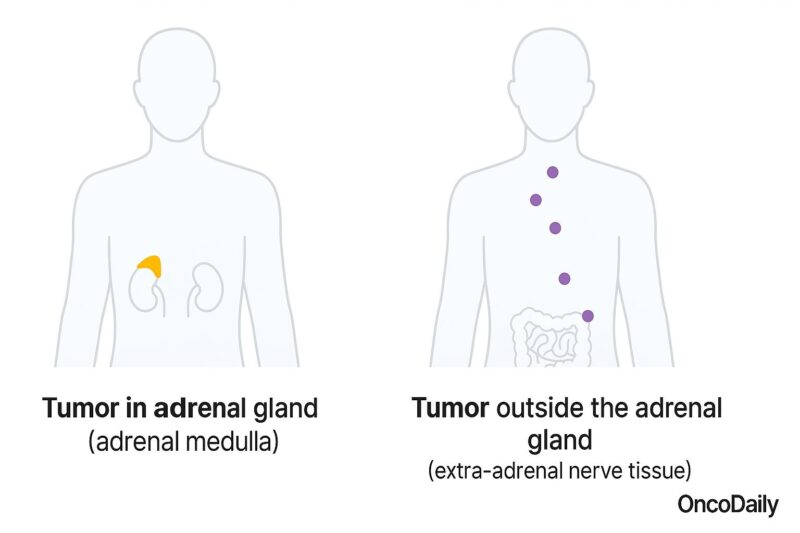
Pheochromocytomas can be classified based on their location, biological behavior, and genetic background. According to their location, they can arise within the adrenal glands (adrenal pheochromocytomas), or less commonly, outside the adrenal glands, along the sympathetic nervous system—these are termed extra-adrenal pheochromocytomas or paragangliomas.
Regarding their biological behavior, most pheochromocytomas are benign, accounting for approximately 85–90% of cases, while malignant pheochromocytomas, which have metastasized to other tissues such as the bones, lungs, or lymph nodes, occur in about 10–15% of patients.
Finally, pheochromocytomas are classified as either sporadic or hereditary. Sporadic cases occur without an underlying genetic cause and typically affect only one adrenal gland. Hereditary pheochromocytomas are associated with genetic syndromes like Multiple Endocrine Neoplasia type 2 (MEN2), Von Hippel–Lindau (VHL) syndrome, Neurofibromatosis type 1 (NF1), or Hereditary Paraganglioma-Pheochromocytoma Syndromes related to mutations in SDHx genes. Classification by these factors is critical for guiding patient management, treatment decisions, and long-term follow-up strategies.
Causes of Pheochromocytoma
Pheochromocytomas arise from chromaffin cells in the adrenal glands, but the exact cause remains unclear in most sporadic cases. However, up to 40% of pheochromocytomas are associated with inherited genetic mutations.
Hereditary forms of pheochromocytoma typically result from mutations in specific genes, including RET (Multiple Endocrine Neoplasia type 2), VHL (Von Hippel–Lindau syndrome), NF1 (Neurofibromatosis type 1), and SDHx genes (Hereditary Paraganglioma-Pheochromocytoma Syndromes). These genetic conditions predispose individuals to develop tumors, often at younger ages and in multiple sites.
Environmental and lifestyle factors are not clearly linked to pheochromocytoma development. Instead, familial genetic syndromes remain the strongest known contributors to tumor occurrence, highlighting the importance of genetic counseling and testing in affected individuals and their families.
Prognosis of Pheochromocytoma
The prognosis for pheochromocytoma depends largely on whether the tumor is benign or malignant, how early it is diagnosed, and the success of surgical removal. Most patients with benign, localized pheochromocytoma who undergo complete surgical resection experience an excellent long-term outlook, with five-year survival rates exceeding 90% (Amar et al., J Clin Endocrinol Metab, 2005).
However, prognosis is less favorable for those with malignant or metastatic disease, or for cases associated with certain genetic mutations such as SDHB, which carry a higher risk of recurrence and metastasis (Fishbein et al., J Clin Oncol, 2017). Malignant pheochromocytoma is defined by the presence of metastases, as histological appearance alone cannot reliably predict behavior (Lenders et al., The Lancet, 2005).
Recurrence can occur even after successful surgery, particularly in patients with hereditary syndromes or bilateral tumors, necessitating lifelong biochemical monitoring (Amar et al., J Clin Endocrinol Metab, 2005). Early detection and advances in targeted therapies, such as belzutifan and tyrosine kinase inhibitors, are gradually improving outcomes for patients with advanced or metastatic disease.
Diagnosis of Pheochromocytoma
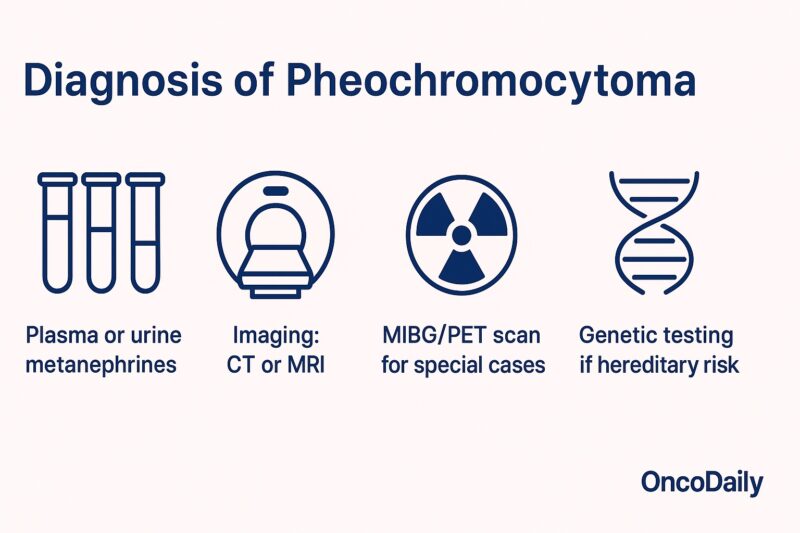
The diagnosis of pheochromocytoma relies on a combination of biochemical testing and imaging studies. The first step is to confirm catecholamine excess. This is best achieved by measuring plasma-free metanephrines or 24-hour urinary fractionated metanephrines and catecholamines. These tests are highly sensitive and specific, as metanephrines are more stable metabolites of catecholamines and less likely to be affected by short-term fluctuations (Eisenhofer et al., JAMA, 2003; Lenders et al., J Clin Endocrinol Metab, 2014).
If laboratory results show elevated levels, imaging is performed to locate the tumor. CT or MRI of the abdomen is usually the initial imaging modality and can identify the majority of adrenal pheochromocytomas. For tumors not found on standard imaging or when there is suspicion of extra-adrenal or metastatic disease, functional imaging such as 123I-MIBG scintigraphy or PET scans may be employed to further characterize the lesion and guide surgical planning (Ilias et al., Endocr Pract, 2011).
Genetic testing is recommended for all patients, particularly those diagnosed at a young age or with bilateral, extra-adrenal, or recurrent tumors, to identify underlying hereditary syndromes. This genetic information is crucial for treatment, prognosis, and family counseling (Neumann et al., N Engl J Med, 2002).
Accurate and timely diagnosis ensures the most appropriate management and can help prevent serious complications from undetected hormone-secreting tumors.
Treatment of Pheochromocytoma
The primary treatment for pheochromocytoma is surgical removal of the tumor. Before surgery, however, careful medical preparation is essential to prevent dangerous fluctuations in blood pressure during the operation. This preparation usually involves starting an alpha-adrenergic blocker (such as phenoxybenzamine or doxazosin) for 7 to 14 days prior to surgery to control hypertension and expand blood volume. Once adequate alpha-blockade is established, a beta-blocker may be added if needed to control tachycardia, but should never be started first, as this can worsen hypertension (Lenders et al., J Clin Endocrinol Metab, 2014).
The surgical approach is typically laparoscopic adrenalectomy for most localized adrenal tumors, offering a minimally invasive option with good outcomes. Open surgery may be necessary for very large, invasive, or malignant tumors (Fishbein et al., J Clin Oncol, 2017).
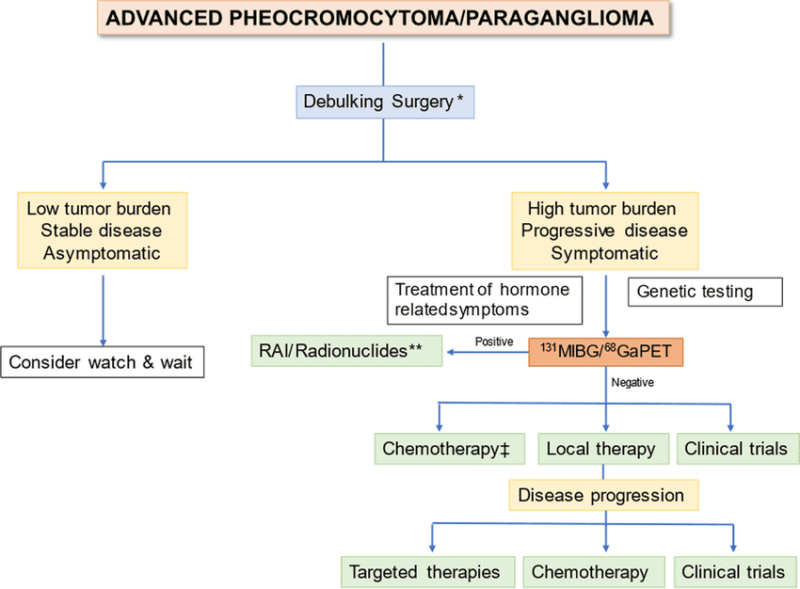
For patients with metastatic or inoperable disease, treatment may include chemotherapy (such as the CVD regimen: cyclophosphamide, vincristine, and dacarbazine), radionuclide therapy (using 131I-MIBG), or targeted therapies like tyrosine kinase inhibitors (Fishbein et al., J Clin Oncol, 2017). Lifelong monitoring and additional therapies may be needed for recurrent or hereditary cases.
Targeted Therapy for Pheochromocytoma
Targeted therapy plays a growing role in the management of advanced or metastatic pheochromocytoma, especially when tumors are inoperable or have spread beyond the adrenal glands. Unlike conventional chemotherapy, targeted therapies are designed to block specific molecular pathways involved in tumor growth and survival.
Several agents have shown promise in clinical practice and research. Tyrosine kinase inhibitors (TKIs), such as sunitinib and pazopanib, target signaling pathways (including VEGF and RET) that are often active in pheochromocytoma and related paragangliomas. These drugs can help slow disease progression and, in some cases, shrink tumors (Fishbein et al., J Clin Oncol, 2017).
Another form of targeted treatment is radionuclide therapy, notably with 131I-MIBG (metaiodobenzylguanidine), which delivers targeted radiation to tumor cells that take up this molecule. MIBG therapy is especially useful for tumors that are MIBG-avid on functional imaging (Ilias et al., Endocr Pract, 2011).
Research is ongoing into newer targeted approaches, including inhibitors of hypoxia-inducible factors (HIFs) and other molecular pathways. The use of targeted therapies is often individualized based on tumor genetics, prior treatments, and the presence of metastatic disease.
Immunotherapy for Pheochromocytoma
Immunotherapy, which harnesses the patient’s immune system to fight cancer, is an emerging area of interest for advanced pheochromocytoma and paraganglioma, particularly in cases that are metastatic or refractory to other treatments. While the mainstay of therapy for pheochromocytoma remains surgical removal and targeted approaches, some patients with aggressive disease may benefit from immunotherapeutic strategies.
Recent studies have investigated the use of immune checkpoint inhibitors—such as pembrolizumab (anti–PD-1 antibody)—in pheochromocytoma and paraganglioma. Early-phase clinical trials and case reports suggest that a subset of patients with progressive or metastatic disease may achieve tumor stabilization or even partial responses with these agents, especially if their tumors have certain molecular features, such as increased PD-L1 expression or high mutational burden (Fishbein et al., J Clin Oncol, 2017; Naing et al., J Clin Endocrinol Metab, 2020).
However, evidence is still limited, and immunotherapy is generally reserved for select patients after more established options have been exhausted, or in the context of clinical trials. Ongoing research aims to clarify which patients may benefit most and how immunotherapy can be best integrated into the overall treatment strategy.
New 2025 Advances in Pheochromocytoma Treatment
As of May 2025, significant progress has been made in the treatment of advanced pheochromocytoma and paraganglioma (PPGL), particularly with the approval of new targeted therapies.
FDA Approval of Belzutifan (Welireg)
On May 14, 2025, the U.S. Food and Drug Administration (FDA) approved belzutifan (Welireg), a hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor, as the first oral therapy for patients aged 12 years and older with locally advanced, unresectable, or metastatic PPGL. This approval was based on the Phase II LITESPARK-015 trial, which demonstrated a 26% objective response rate and a median duration of response of 20.4 months.
Clinical Impact
Belzutifan’s approval offers a new treatment option for patients with advanced PPGL, providing a convenient oral therapy that can reduce tumor burden and potentially decrease the need for antihypertensive medications. In the LITESPARK-015 trial, 32% of patients on baseline antihypertensive medications reduced their medication use by at least 50% for six months.
Considerations and Side Effects
While belzutifan represents a significant advancement, it is important to monitor for potential side effects, including anemia, fatigue, and hypoxia. These adverse events are generally manageable with appropriate dose adjustments and supportive care.
The approval of belzutifan marks a pivotal moment in the management of advanced PPGL, offering hope for improved outcomes in this rare and challenging disease.

Read More About Belzutifan(Welireg) Approval on Oncodaily
Living After Pheochromocytoma: Follow-Up and Monitoring
Life after pheochromocytoma surgery usually brings significant improvement in symptoms and blood pressure control, especially when the tumor is benign and completely removed. However, long-term follow-up remains essential for all patients, as recurrence and the development of new tumors are possible, particularly in those with hereditary syndromes or bilateral disease (Amar et al., J Clin Endocrinol Metab, 2005).
Ongoing surveillance typically includes annual measurements of plasma or urinary metanephrines to detect early recurrence. Imaging studies may also be recommended if symptoms recur or if biochemical tests are abnormal (Lenders et al., J Clin Endocrinol Metab, 2014).
Patients with hereditary syndromes require lifelong monitoring for pheochromocytoma as well as screening for associated conditions, such as medullary thyroid carcinoma in MEN2 or renal cell carcinoma in VHL syndrome (Neumann et al., N Engl J Med, 2002). Genetic counseling is recommended for patients and their families to guide screening and management.
Emotional and psychological support can also play an important role, as patients may experience anxiety about recurrence or ongoing health concerns. Education about symptoms that should prompt urgent medical attention—such as severe headaches, palpitations, or new episodes of hypertension—is crucial for long-term wellbeing.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
FAQ
What is pheochromocytoma?
Pheochromocytoma is a rare tumor that develops in the adrenal glands and causes excess production of adrenaline and noradrenaline, leading to high blood pressure and other symptoms.
What are the common symptoms of pheochromocytoma?
Common symptoms include sudden episodes of high blood pressure, severe headaches, rapid heartbeat, excessive sweating, anxiety, and palpitations.
How is pheochromocytoma diagnosed?
Diagnosis involves measuring catecholamines or metanephrines in blood or urine, followed by imaging tests like CT or MRI scans to locate the tumor.
Is pheochromocytoma cancerous?
Most pheochromocytomas are benign, but about 10–15% can be malignant and may spread to other parts of the body.
What causes pheochromocytoma?
While most cases occur randomly, up to 40% are linked to inherited genetic syndromes such as MEN2, VHL, NF1, or SDHx mutations.
How is pheochromocytoma treated?
The main treatment is surgical removal of the tumor, with careful medical preparation to control blood pressure before surgery. Targeted therapy or other treatments may be used for advanced cases.
Can pheochromocytoma recur after surgery?
Yes, recurrence is possible, especially in patients with hereditary forms. Lifelong follow-up with regular biochemical testing is recommended.
What are the risks if pheochromocytoma is not treated?
Untreated pheochromocytoma can cause life-threatening complications such as hypertensive crises, stroke, heart attack, or heart failure.
Should family members of someone with pheochromocytoma be tested?
If the tumor is linked to a hereditary syndrome, genetic counseling and testing for family members may be recommended.
What are the latest advances in pheochromocytoma treatment?
Recent advances include targeted therapies like tyrosine kinase inhibitors and, as of 2025, FDA approval of new drugs such as belzutifan for advanced or metastatic cases.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
