Immunotherapy has transformed the treatment of melanoma—once one of the most challenging skin cancers to treat in its advanced stages. By harnessing the power of the immune system, therapies like PD-1 and CTLA-4 checkpoint inhibitors have significantly improved survival rates, offering durable responses that were once unimaginable with conventional treatments.
Today’s research is pushing beyond established therapies—into neoadjuvant approaches, next-generation combinations, personalized immunotherapies, and biomarkers of resistance. These advances are happening not just in laboratories, but in real-world clinical trials that pave the way for tomorrow’s standard of care.
Clinical trials are the engine of innovation in oncology. They offer patients early access to promising treatments and provide essential data to improve care for future generations. Especially in a fast-evolving field like melanoma immunotherapy, participation in trials is critical—not just to validate new strategies, but to accelerate how quickly they reach those who need them most.
Neoadjuvant Immunotherapy with T-VEC + Pembrolizumab in Stage III Melanoma
A Phase 2 clinical trial is exploring a promising neoadjuvant approach for patients with resectable Stage III cutaneous melanoma and clinically detectable lymph node metastases. The study is testing a combination of two immunotherapies—pembrolizumab (Keytruda) and Talimogene Laherparepvec (T-VEC)—given before surgery.
In this single-arm, open-label study, patients receive:
-
Pembrolizumab via IV every 3 weeks for 6 months
-
T-VEC through direct injection into the tumor (palpable lymph nodes) on the same schedule
After this 6-month preoperative treatment, patients undergo complete lymph node dissection. Post-surgery, pembrolizumab is continued as adjuvant therapy for up to one year.
The main goal is to assess pathologic complete response (pCR)—whether the cancer is completely eliminated from the lymph nodes at the time of surgery. Researchers are also closely monitoring the safety and tolerability of this combination over time, with follow-up lasting up to 5 years.
The AXIOM Trial Testing Radiotherapy and Immunotherapy in Melanoma
The AXIOM trial is a multicenter, randomized, non-comparative Phase II clinical study investigating whether stereotactic body radiotherapy (SBRT) can enhance the effectiveness of immunotherapy in patients with treatment-naïve extracranial oligometastatic melanoma.
The rationale behind combining SBRT and immunotherapy stems from increasing evidence of their synergistic effects. Radiation has the potential to convert immunologically “cold” tumors into “hot,” promoting immune activation by altering cytokine balance, enhancing antigen presentation, and improving immune cell recruitment to the tumor microenvironment. While both therapies are already used individually in advanced melanoma, this study aims to determine whether giving them concurrently from the start can lead to better outcomes.
Participants will be randomized in a 2:1 ratio to receive either:
-
SBRT concurrently with immunotherapy, or
-
Immunotherapy alone, which reflects the current standard of care.
The study targets patients with 1–5 extracranial metastases and evaluates whether adding SBRT upfront can improve overall survival, progression-free survival, local control, and overall response rate without increasing toxicity.
SBRT is delivered in fractions, precisely targeting metastatic sites while sparing healthy tissue. Immunotherapy will consist of checkpoint inhibitors, although the specific agents used may vary per center protocol.
The primary endpoint is overall survival measured at multiple time points (6 months, 1, 2, 3, and 5 years). Secondary endpoints include:
-
Progression-free survival (overall and for new lesions only)
-
Overall response rate
-
Local control of baseline metastases
-
Treatment safety and tolerability using CTCAE v5.0
-
Use of immunosuppressive agents for toxicity management
-
Quality of life using validated QOL instruments
-
Use of salvage radiotherapy in the immunotherapy-only arm
The Grand SLAM Trial Testing Shorter Adjuvant Immunotherapy in Melanoma
The Grand SLAM trial is a Phase III, international, randomized study investigating whether 6 months of adjuvant PD-1 immunotherapy is as effective as the current 12-month standard for patients with high-risk cutaneous melanoma (Stage IIb–IV) after surgery.
Checkpoint inhibitors like nivolumab and pembrolizumab are routinely given for one year, but this duration was never optimized—and brings added toxicity, cost, and burden. Grand SLAM aims to show that shorter treatment may maintain efficacy while improving patient quality of life and reducing healthcare resource use.
Patients are randomized to receive either 6 or 12 months of therapy. The primary endpoints are relapse-free survival (RFS) and distant metastasis-free survival (DMFS) at 2 years. A total of 1,792 patients will be followed for up to 5 years, with interim analysis planned after 2/3 enrollment.
If successful, Grand SLAM could redefine the duration of adjuvant therapy in melanoma—making treatment safer, shorter, and more sustainable.
TIL 1383I Trial Using Engineered T Cell Therapy Against Advanced Melanoma
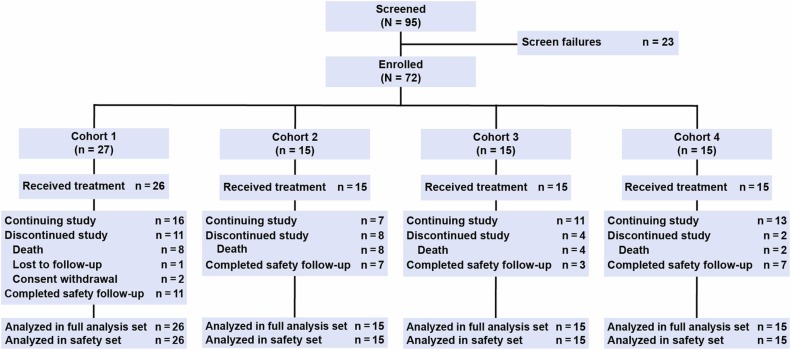
This Phase I dose-escalation study, funded by the National Cancer Institute (NCI), explores a novel adoptive cell therapy approach for advanced melanoma using autologous T cells genetically modified with the TIL 1383I T-cell receptor (TCR).
The trial tests escalating doses of TIL 1383I TCR-transduced T cells in three patient cohorts to determine the maximum tolerated dose and define a recommended Phase II dose. The engineered T cells are delivered via a single infusion, supported by low-dose IL-2, and include both CD4+ and CD8+ subsets.
-
Cohort 1: 7.5 x 10⁶ cells/kg
-
Cohort 2: 2.5 x 10⁷ cells/kg
-
Cohort 3: 7.5 x 10⁷ cells/kg
Each group is monitored for dose-limiting toxicities (DLTs), adverse events (including vision and hearing changes), and early signs of tumor response (via imaging and physical exam) over a 1-year follow-up.
LNS8801-MEL Study Testing Targeted Therapy Plus Immunotherapy in Advanced Melanoma
This multicenter clinical trial evaluates whether LNS8801, a GPER agonist, can effectively and safely treat patients with unresectable, treatment-refractory melanoma who have progressed after immune checkpoint inhibitor therapy. The focus is on patients with the C/C GPER genotype, which occurs in roughly 55% of melanoma cases.
A total of 135 patients will be randomly assigned to one of three treatment arms:
-
Arm 1: LNS8801 alone – taken as a 125 mg tablet daily for up to 2 years.
-
Arm 2: LNS8801 combined with pembrolizumab – patients will receive daily LNS8801 plus IV pembrolizumab every 3 weeks for up to 2 years.
-
Arm 3 (Physician’s Choice): Patients will receive standard-of-care treatment, either chemotherapy (dacarbazine or temozolomide) or immunotherapy (e.g., pembrolizumab, nivolumab/relatlimab, or nivolumab/ipilimumab), as chosen by the treating physician.
The study will assess progression-free survival (PFS), overall response rate (ORR), duration of response (DOR), disease control rate (DCR), and overall survival (OS), alongside safety and tolerability metrics. Quality-of-life scores and metabolic health markers like HbA1C and lipids will also be monitored.
Imaging will be conducted regularly to track tumor progression. Patients may continue treatment beyond disease progression if deemed beneficial. Safety follow-ups will continue after the last dose (30 or 90 days depending on therapy type), with long-term OS tracked for up to 5 years.
IL4I1 as a Biomarker of Melanoma Resistance and Prognosis
This prospective study explores the role of IL4I1+ immune cells as a predictive and prognostic marker in patients with cutaneous melanoma. IL4I1 (Interleukin-4-induced gene 1) is an immunosuppressive enzyme involved in amino acid metabolism, and it may contribute to immune evasion and treatment resistance.
Researchers aim to:
-
Quantify IL4I1+ cells in blood, primary tumors, sentinel lymph nodes, and metastases
-
Compare expression patterns before and after treatment with immunotherapy (anti-PD1 ± anti-CTLA4) or targeted therapy (BRAF ± MEK inhibitors)
-
Correlate IL4I1 levels with disease progression speed and resistance to treatment
The study includes three patient groups:
- Patients with thin melanomas (≤1mm Breslow) monitored long-term
- Patients with thick melanomas (≥3mm Breslow) monitored for recurrence
- Patients with advanced stage III/IV melanoma receiving systemic therapies
Blood samples and tumor biopsies are collected at baseline, after 3 months, and at 1 year or upon progression. The study investigates whether higher IL4I1 expression is linked to faster relapse or resistance to immunotherapy, with potential implications for future biomarker development and drug targeting.
NEOMEL Study of Neoadjuvant Immunotherapy in Operable Metastatic Melanoma
This ongoing observational study evaluates the real-life effectiveness and safety of neoadjuvant immunotherapy in patients with operable stage III or IV metastatic melanoma.
Patients are treated with anti-PD1 monotherapy or anti-PD1 combined with anti-CTLA-4 prior to surgery. The study aims to assess:
-
Objective response rates on imaging
-
Histological complete response after surgery
-
Event-free survival at 12 months, including events such as disease progression that prevents surgery, delays in starting adjuvant treatment, recurrence, or death
-
Disease-free survival, metastasis-free survival, and overall survival
-
Toxicity profiles, focusing on grade 3–4 adverse events (CTCAE v5.0)
Neoadjuvant Dual Immune Checkpoint Blockade in High-Risk Stage II Melanoma
A new phase 2 clinical trial is testing whether giving immunotherapy before surgery can improve outcomes for people with high-risk stage IIB and IIC melanoma. These patients have a significant chance of their cancer coming back after surgery—sometimes even higher than patients with stage III disease—yet the current standard of care is just observation.
In this study, patients will receive two doses of a combination immunotherapy (relatlimab + nivolumab) before surgery. This combination targets two immune checkpoints (LAG-3 and PD-1) and has already shown promise in more advanced stages of melanoma. The goal is to stimulate the immune system early, when the tumor is still present, and increase the chance of a lasting response.
After the two doses, patients will have surgery and sentinel lymph node biopsy. If there’s no or very little cancer left in the tissue (a strong response), they will go on regular monitoring. If there’s still a moderate amount of cancer, they’ll get additional 11 cycles of immunotherapy after surgery.
Researchers will look at how many patients respond well to the pre-surgery treatment, how many stay cancer-free over time, and how well the treatment is tolerated. They’ll also analyze blood, tissue, and even gut microbiome samples to look for biomarkers that could predict who benefits most.
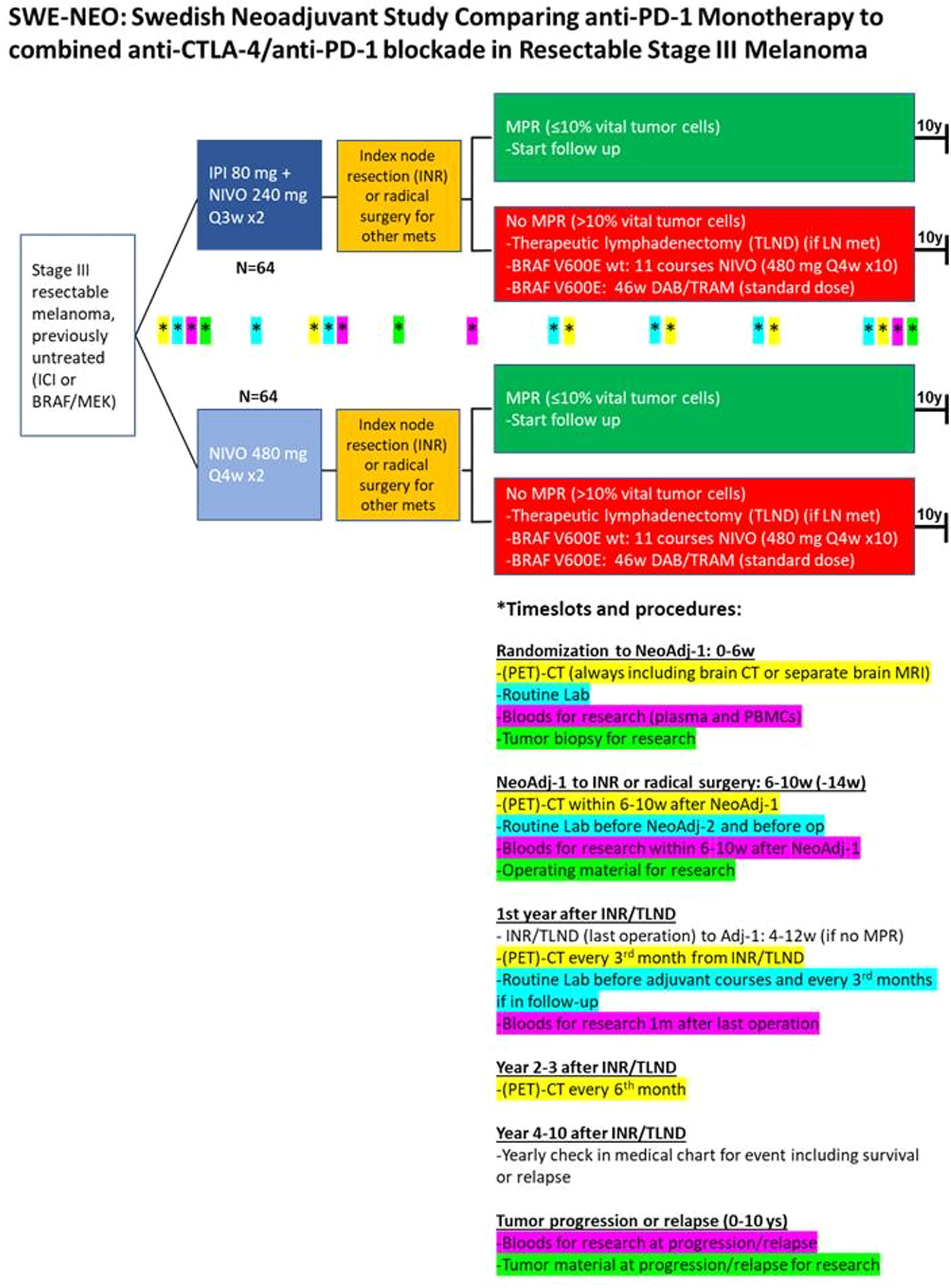
SWE-NEO Trial: Comparing Neoadjuvant PD-1 Monotherapy vs Dual Checkpoint Blockade in Resectable Stage III Melanoma
A new phase III trial in Sweden, SWE-NEO, is set to address an important gap in melanoma research: comparing PD-1 monotherapy to PD-1 + CTLA-4 combination therapy in the neoadjuvant setting for resectable stage III melanoma. While recent studies like SWOG S1801 and NADINA have already confirmed that giving immunotherapy before surgery improves outcomes compared to after surgery, they used different regimens—PD-1 monotherapy in SWOG and dual immunotherapy in NADINA—making direct comparisons impossible.
This head-to-head trial will enroll 128 patients and randomize them to receive either nivolumab alone or a combination of nivolumab and ipilimumab prior to surgery. After surgery, only patients who show limited response in the tumor will receive adjuvant therapy: either nivolumab or, for those with a BRAF mutation, a BRAF/MEK inhibitor.
The study will closely monitor event-free survival, relapse-free survival, distant metastasis-free survival, and overall survival over five years. It also includes in-depth biomarker analyses from sequential blood and tumor samples to explore predictors of response and toxicity. Safety will be carefully tracked, especially given the known higher toxicity risk with dual immune checkpoint blockade.
LIMIT Melanoma Trial on Hydroxychloroquine with Nivolumab or Nivolumab Ipilimumab in Advanced Melanoma
This phase 1/2 clinical trial investigates the safety, tolerability, and effectiveness of combining hydroxychloroquine (HCQ) with immunotherapy in patients with advanced or metastatic melanoma. The trial is divided into three parts:
-
Phase 1a evaluates the maximum tolerated dose (MTD) and safety of HCQ with nivolumab.
-
Phase 1b evaluates the MTD and safety of HCQ with both nivolumab and ipilimumab.
-
Phase 2 assesses how well HCQ combined with nivolumab works in treating melanoma (objective response rate).
Patients will receive either dual or triple immunotherapy (nivolumab ± ipilimumab) plus oral HCQ. The trial will monitor response, survival, and side effects over time. Recruitment is ongoing in the United States.
Written by Toma Oganezova, MD, Editor-in-Chief of OncoDaily IO