Pluvicto (lutetium Lu 177 vipivotide tetraxetan) is a targeted radioligand therapy used for treating advanced prostate cancer. It delivers radiation directly to cancer cells while sparing healthy tissue.
The FDA first approved Pluvicto on March 23, 2022, for patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) who had previously received androgen receptor inhibitors and taxane-based chemotherapy. This approval was based on the VISION trial, which showed improved overall survival and radiographic progression-free survival.
On March 28, 2025, the FDA expanded its approval, allowing its use earlier in treatment for eligible patients who had received an androgen receptor pathway inhibitor but had not yet undergone chemotherapy. This expansion gives patients access to Pluvicto sooner, offering a targeted treatment option before chemotherapy becomes necessary.
Which Company Produces Pluvicto?
Pluvicto is produced by Novartis, a leading global pharmaceutical company headquartered in Switzerland. Novartis specializes in innovative medicines, including oncology, cardiology, immunology, and neuroscience. The company is known for its research-driven approach, developing breakthrough therapies such as Pluvicto for prostate cancer and Kymriah for blood cancers.
How Does Pluvicto Work?
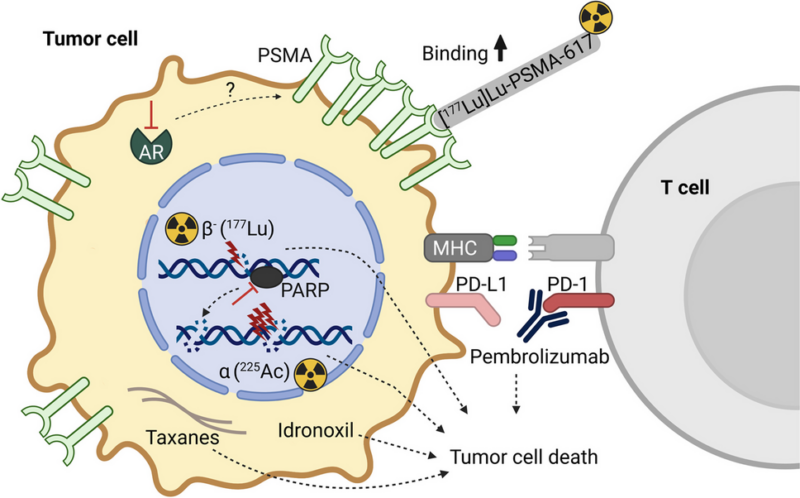
Pluvicto is a targeted therapy designed to deliver radiation directly to cancer cells expressing PSMA, a protein found in high levels on prostate cancer cells. The treatment combines Lutetium-177 (177Lu), a radioactive isotope, with a small molecule that specifically binds to PSMA.
Once injected, Pluvicto attaches to the cancer cells and delivers a focused radiation dose, helping to shrink tumors and extend patient survival while minimizing damage to nearby healthy tissue.
What Cancers Is Pluvicto Approved to Treat?
The FDA first approved Pluvicto on March 23, 2022, for patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) who had already been treated with hormone therapy (androgen receptor inhibitors) and chemotherapy (taxane-based). This approval was based on the VISION trial, which showed that Pluvicto helped patients live longer and delayed the spread of cancer seen on scans.
In 2025, the FDA expanded its approval. Now, Pluvicto can also be used earlier in treatment—before patients receive chemotherapy—if their cancer is PSMA-positive.

Learn more about Prostate Cancer: What patients should know? on OncoDaily.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Pluvicto was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
Efficacy and Results from Clinical Trials
Pluvicto has been extensively studied in clinical trials to determine its effectiveness in treating advanced prostate cancer. Two major studies, the PSMAfore and VISION trials, provide strong evidence supporting its use.
The PSMAfore trial (NCT04689828) focused on patients whose cancer had progressed despite treatment with androgen receptor pathway inhibitors (ARPIs) but who had not yet received chemotherapy. The goal was to see if Pluvicto could delay cancer progression and improve survival outcomes.
Patients receiving Pluvicto had a median radiographic progression-free survival (rPFS) of 9.3 months, meaning their cancer did not worsen for nearly four months longer than those who received standard ARPI treatment, which had an rPFS of 5.6 months.
The median overall survival (OS) was 24.5 months for Pluvicto-treated patients, compared to 23.1 months for those receiving ARPIs alone. A significant 57.6% of patients experienced a PSA decline of 50% or more, indicating a strong treatment response.
The VISION trial (NCT03511664) included patients with metastatic castration-resistant prostate cancer (mCRPC) who had already been treated with both ARPIs and taxane-based chemotherapy. It tested whether adding Pluvicto to standard care could further improve outcomes.
Patients treated with Pluvicto had a median rPFS of 8.7 months, compared to just 3.4 months for those receiving standard care alone. The median overall survival (OS) was 15.3 months in the Pluvicto group, compared to 11.3 months in the control group. This means Pluvicto reduced the risk of death by 38%. Additionally, 46% of patients saw their PSA levels drop by 50% or more, a key marker of treatment effectiveness.
Read more about Immunotherapy for Prostate Cancer on OncoDaily.
What Do These Results Mean ?
These trials show that Pluvicto can significantly delay cancer progression and help patients live longer. Importantly, it works even for patients who have already received multiple prior treatments, providing a much-needed option for those with advanced prostate cancer. If you or a loved one has PSMA-positive metastatic prostate cancer, discussing Pluvicto with your doctor could help determine whether this treatment is a suitable option.
Recommended Dosage of Pluvicto?
Pluvicto is given as an IV injection for men with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) who have already been treated with hormone therapy and may have had chemotherapy or are delaying it. The usual dose is 200 mCi (7.4 GBq) given every 6 weeks, for up to 6 doses, or until the cancer worsens or side effects become too severe.
How Pluvicto Is Given?
Pluvicto is given through a vein using a syringe or infusion pump. The drug is carefully handled by medical staff trained in using radioactive medicine. The dose is checked before and after each treatment. After giving the drug, the IV line is flushed with saline. Any unused medicine is safely disposed of following strict safety rules.Pluvicto is stored in special containers to protect from radiation and must be kept below 30°C (86°F), not frozen.
Pluvicto Side Effects and Management
Pluvivto, like many medications, can cause side effects. Some of these are common and can be managed easily, while others are less common but may require more attention.
Common Side Effects
One of the most common side effects is fatigue, which can make you feel extremely tired. The best way to manage this is by getting plenty of rest, staying hydrated, and engaging in light exercise when you feel up to it. Another common issue is dry mouth, which can be uncomfortable. This can be relieved by using saliva substitutes and drinking enough fluids throughout the day. Nausea and vomiting can also occur, but these can be treated with anti-nausea medications and adjusting your diet to include bland foods that are easier to digest.
Anemia, or a low red blood cell count, can happen as a result of the medication. This is typically managed with blood transfusions or medications to help boost your red blood cell production. A decreased appetite is another possible side effect, which may make it difficult to eat enough. In this case, it’s helpful to eat small, high-calorie meals throughout the day to maintain nutrition. Constipation is also common, but can be managed by increasing fiber intake, staying hydrated, and using laxatives if needed.
Less Common Side Effects
There are also some less common, but more serious, side effects. Myelosuppression, a condition where the bone marrow produces fewer blood cells, may occur and requires regular blood tests to monitor your levels. If necessary, your doctor may adjust the dosage of your medication. Renal toxicity, which can affect kidney function, is another potential concern. This is managed by making sure you stay well-hydrated and by monitoring your kidney function with regular tests.
Electrolyte imbalances, such as low potassium or sodium levels, can also happen. These can be monitored through blood tests and corrected with supplements if needed. Lastly, gastrointestinal issues, like diarrhea or stomach discomfort, are possible but can often be controlled through dietary adjustments and medications.
It’s important to talk to your healthcare team if you experience any of these side effects, as they can help you manage them and adjust your treatment plan to ensure your well-being.

What Should You Avoid During Treatment?
During treatment, there are a few important things to avoid in order to ensure the patient’s safety and the effectiveness of the medication. First, radiation exposure, especially to pregnant women and children, should be avoided. The patient should take precautions to minimize close contact with pregnant individuals and young children during this time.
The patient should also avoid taking live vaccines unless they have consulted with their doctor. Live vaccines can interact with the treatment, so it is essential to discuss this with the healthcare provider before receiving any vaccinations.
Another important consideration is that the patient should avoid administering other intravenous (IV) medications through the same catheter used for the treatment. This helps prevent complications and ensures the medication is delivered properly.
Finally, the patient should reduce activities that could increase the risk of infection or bleeding, such as contact sports or activities that could lead to injury. The immune system may be weakened during treatment, so it is important to take steps to protect against infections or injury.
It is always recommended that the patient communicates with their healthcare team if they are unsure about any activities or medications to avoid. The healthcare team is there to guide the patient through the treatment process and ensure they remain as safe and healthy as possible.
How Well Pluvicto Works Over Time?
Pluvicto is a treatment for advanced prostate cancer that keeps working even after other therapies stop helping. In a major study, it delayed cancer growth for about 9.3 months, compared to 5.6 months with hormone therapy alone. Early results also show it may help patients live longer, and research is ongoing to better understand its long-term benefits.
Looking Ahead: The Future of Pluvicto
Future research is focused on expanding Pluvicto’s use in other cancers, such as renal cell carcinoma, and improving outcomes through combination therapies.
Pluvicto represents a significant advancement in prostate cancer treatment, offering a promising option for patients with PSMA-positive mCRPC. Ongoing trials continue to explore its potential across different settings and cancer types.
What Other Trials Are Ongoing?
Several ongoing clinical trials are investigating Pluvicto in different treatment settings to explore its potential benefits. The ENZA-p Phase 2 study evaluated Pluvicto in combination with Xtandi (enzalutamide) and found that it improved PSA progression-free survival and response rates. Another Phase 2 trial is studying Pluvicto in patients with high-volume metastatic hormone-sensitive prostate cancer (mHSPC), where combining it with docetaxel has shown better outcomes compared to docetaxel alone.
The PRINCE trial is exploring Pluvicto in combination with pembrolizumab, an immunotherapy drug, and has shown promising PSA response rates in patients with metastatic prostate cancer. The CATCH-177 study (NCT06738303) is comparing a combination of cabazitaxel and carboplatin to Pluvicto in metastatic castration-resistant prostate cancer (mCRPC), which could help determine the most effective treatment approach.
Lastly, the STARLiT trial (NCT06574880) is investigating whether Pluvicto can replace traditional androgen deprivation therapy (ADT) when combined with radiation therapy.
These ongoing studies aim to expand the potential use of Pluvicto in different stages of prostate cancer, offering new hope for patients looking for improved treatment options.
If you’re a healthcare provider, access the professional version here.
