Pluvicto (lutetium Lu 177 vipivotide tetraxetan) is a targeted radioligand therapy used to treat advanced prostate cancer by delivering radiation directly to prostate-specific membrane antigen (PSMA)-positive cancer cells, limiting damage to healthy tissue. On March 28, 2025, the U.S. Food and Drug Administration (FDA) approved an expanded use of Pluvicto for patients with metastatic castration-resistant prostate cancer (mCRPC) who have previously been treated with an androgen receptor pathway inhibitor (ARPI) and are considered appropriate candidates to delay chemotherapy.
This update, announced by Novartis, allows eligible patients to access Pluvicto earlier in their treatment course. This article will explore how Pluvicto works, who it’s for, how it’s given, possible side effects, and what patients can expect during treatment.
Which company produced Pluvicto?
Pluvicto (lutetium Lu 177 vipivotide tetraxetan) is produced by Novartis, a leading global pharmaceutical company headquartered in Switzerland. Novartis specializes in innovative medicines, including oncology, cardiology, immunology, and neuroscience. The company is known for its research-driven approach, developing breakthrough therapies such as Pluvicto for prostate cancer and Kymriah for blood cancers. With a strong focus on precision medicine, Novartis continues to advance treatments that improve patient outcomes worldwide.
How does Pluvicto work?
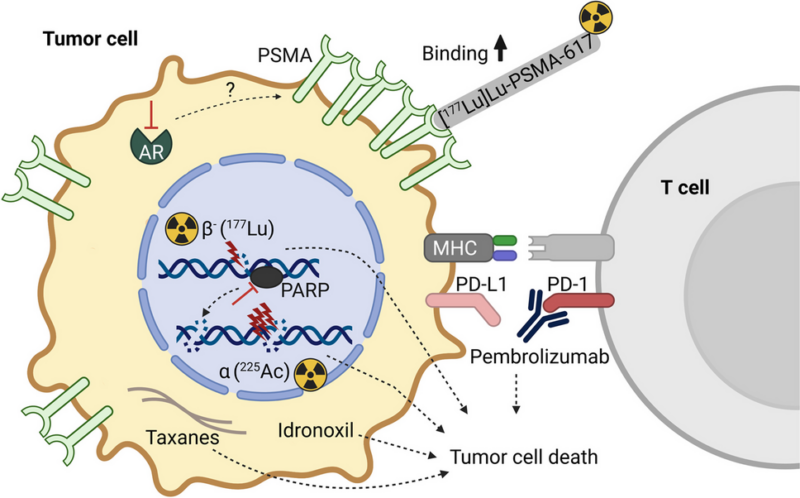
Pluvicto is an advanced targeted therapy designed to deliver radiation directly to cancer cells expressing PSMA, a protein that is often found in high levels on prostate cancer cells. The treatment combines Lutetium-177 (177Lu), a radioactive isotope, with a small molecule that specifically binds to PSMA.
Once injected, Pluvicto attaches to the cancer cells and delivers a focused radiation dose. This helps to shrink the tumors and extend patient survival while minimizing damage to nearby healthy tissue.
Pluvicto works by targeting prostate cancer cells, particularly in metastatic castration-resistant prostate cancer (mCRPC), where PSMA is present in high amounts. After binding to the PSMA on the cancer cells, Pluvicto delivers a dose of beta radiation directly to them. This radiation causes significant DNA damage, preventing the cancer cells from replicating and ultimately leading to their death, which results in the shrinking or elimination of tumors.
Since Pluvicto is designed to target only PSMA-positive cells, healthy tissue that does not express this protein is largely spared from the radiation, making it a more precise treatment option compared to traditional chemotherapy, which affects both cancerous and healthy cells.
What Cancers Is Pluvicto Approved to Treat?
The FDA first approved Pluvicto on March 23, 2022, for patients with PSMA-positive mCRPC who had previously received androgen receptor inhibitors and taxane-based chemotherapy. This approval was based on the VISION trial, which showed improved overall survival and radiographic progression-free survival.
Pluvicto is approved to treat PSMA-positive metastatic castration-resistant prostate cancer (mCRPC). Initially approved for patients who had received an androgen receptor pathway inhibitor and taxane-based chemotherapy, its approval was expanded in 2025 to allow earlier use in patients who have not yet undergone chemotherapy.
What research is behind the approval?
Pluvicto’s approval is supported by two major phase 3 trials: VISION and PSMAfore. VISION showed survival benefits in patients previously treated with both ARPI and chemotherapy, while PSMAfore demonstrated its effectiveness earlier in treatment—after ARPI but before chemotherapy. These studies confirmed Pluvicto’s role across multiple stages of advanced prostate cancer.
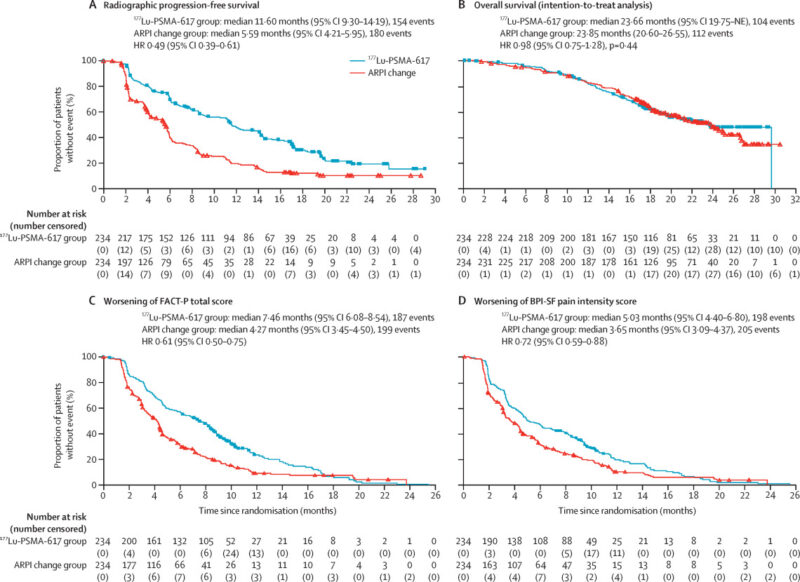
The PSMAfore, a phase 3 randomized trial, published in The Lancet Oncology in 2024 evaluated [177Lu]Lu-PSMA-617 (Pluvicto) in patients with PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) who had progressed after one prior androgen receptor pathway inhibitor (ARPI) but had not yet received chemotherapy. Conducted across 74 sites in Europe and North America, the study included 468 taxane-naive patients randomized to receive either 177Lu-PSMA-617 or a change in ARPI (abiraterone or enzalutamide). Crossover to 177Lu-PSMA-617 was allowed after radiographic progression in the control group.
The trial showed that Pluvicto significantly prolonged radiographic progression-free survival (rPFS): 11.6 months with Pluvicto versus 5.6 months with ARPI change (hazard ratio [HR] 0.49; p<0.0001). In the earlier analysis, rPFS was 9.3 vs. 5.6 months (HR 0.41). The safety profile was also favorable, with fewer grade 3–5 adverse events in the Pluvicto group (36% vs. 48%), and no treatment-related deaths reported. These results supported the FDA’s March 2025 approval of Pluvicto for earlier use in treatment—before chemotherapy—offering a well-tolerated, effective option for eligible patients.
You can find more information about Pluvicto’s approval on OncoDaily.
The VISION trial, which was published in The New England Journal of Medicine in 2021, evaluated Pluvicto in a more advanced patient population, those who had previously been treated with both ARPIs and taxane-based chemotherapy. This trial demonstrated that Pluvicto significantly outperformed standard care in terms of both rPFS and OS.
The median rPFS was 8.7 months for the Pluvicto group, compared to just 3.4 months for the control group. Pluvicto also significantly improved OS, with a median of 15.3 months in the treatment arm, compared to 11.3 months in the control arm, representing a 38% reduction in the risk of death. Additionally, 46.0% of patients in the Pluvicto arm achieved a ≥50% decline in PSA levels, a clear marker of treatment success.

Both trials underscore the efficacy of Pluvicto in delaying disease progression and improving survival outcomes in mCRPC, whether used earlier in the treatment sequence (as in PSMAfore) or in patients with more advanced disease who have failed prior therapies (as in VISION). These findings have cemented Pluvicto’s role as a critical treatment option in the management of mCRPC.
Highlights on Lu-PSMA-617 in taxane-naive patients with mCRPC: The PSMAfore study
Pluvicto Combinations and Treatment Outcomes
The results of the ENZA-p phase 2 study were presented at the 2023 ESMO Congress. The study showed that Pluvicto (Lu-PSMA-617) combined with Xtandi (enzalutamide) improved PSA progression-free survival (PSA-PFS) in metastatic castration-resistant prostate cancer (mCRPC) patients compared to Xtandi alone. The combination therapy had a median PSA-PFS of 13 months versus 7.8 months with Xtandi alone, and radiographic PFS was 16 months vs. 12 months.
The combination also showed better PSA response rates, with 93% achieving a 50% PSA decrease and 78% a 90% decrease, compared to 68% and 37% with Xtandi alone. Serious side effects were similar in both groups.
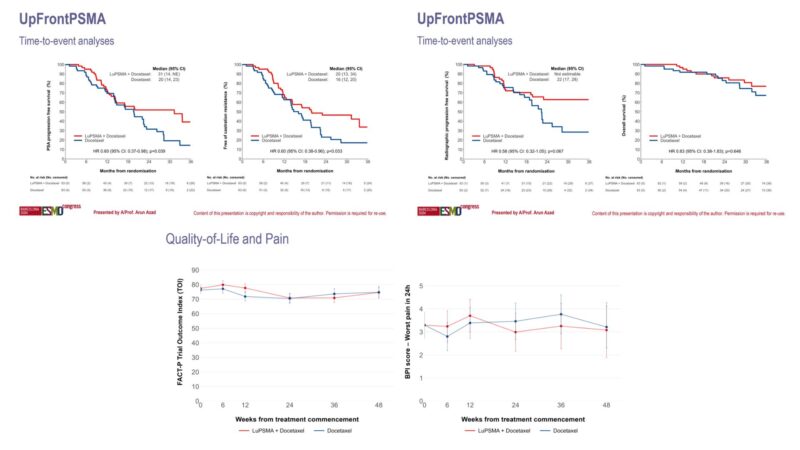
The 2024 ESMO Congress presented results from a phase 2 trial evaluating 177Lu-PSMA-617 (LuPSMA) followed by docetaxel (D) in de novo high-volume metastatic hormone-sensitive prostate cancer (mHSPC). Patients receiving LuPSMA + docetaxel (Arm A) showed significantly better outcomes than those receiving docetaxel alone (Arm B), with higher undetectable PSA rates at 48 weeks (41% vs. 16%) and longer PSA-PFS (30 vs. 21 months), freedom from castration resistance (20 vs. 16 months), and radiographic PFS. The sequential use of LuPSMA followed by docetaxel improved clinical outcomes without increased toxicity.
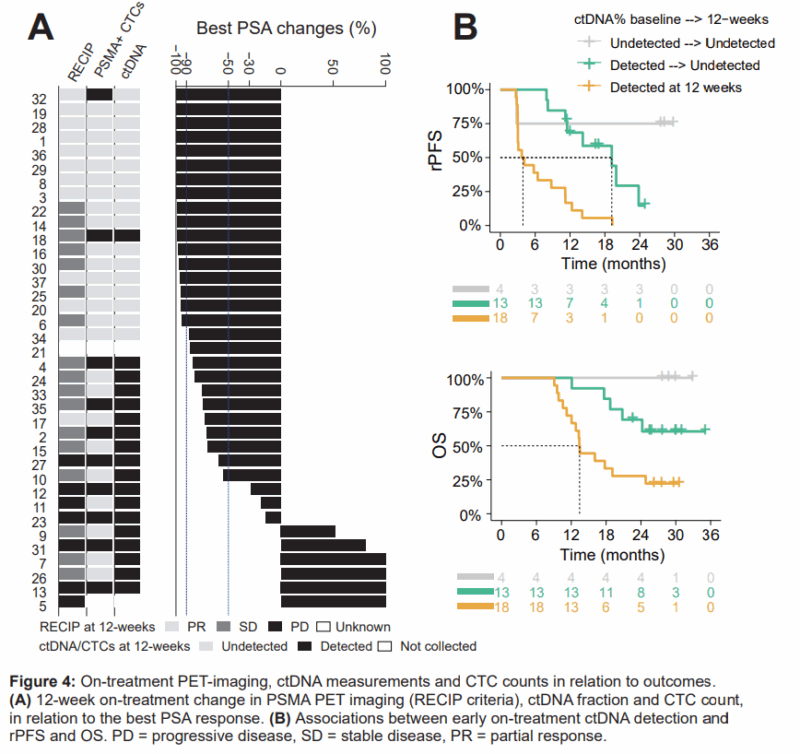
At the 2024 ESMO Congress, the PRINCE trial presented promising results for LuPSMA combined with pembrolizumab in metastatic prostate cancer. An exploratory analysis assessed ctDNA and PSMA-PET as predictors of response. Baseline ctDNA% and PSMA-SUVmean were correlated with radiographic PFS (rPFS), showing that lower ctDNA% and higher PSMA-SUVmean were associated with longer rPFS. Patients with undetected ctDNA after 12 weeks had a higher PSA90 response rate (94%) compared to those with persistent ctDNA (6%).
The study suggests that PSMA SUVmean and ctDNA% may improve rPFS prognostication and highlights the potential of early ctDNA measurements to predict treatment response.
Pluvicto side effects and its management
Pluvicto is a promising treatment for metastatic castration-resistant prostate cancer (mCRPC), but like all medications, it can cause side effects. Managing these side effects is essential for ensuring patients receive the full benefit of the treatment while minimizing discomfort and complications.
Common Side Effects and Their Management
Fatigue is one of the most frequently reported side effects. Patients may experience tiredness or weakness. Managing fatigue involves balancing rest with light physical activity, such as walking or light exercises, to avoid deconditioning. Staying hydrated and consuming a nutritious diet also help in managing fatigue.
Dry mouth is another common side effect, which occurs due to reduced saliva production. This can lead to discomfort, difficulty swallowing, and an increased risk of oral infections. Managing dry mouth involves using saliva substitutes, staying hydrated, chewing sugar-free gum, or sucking on sugar-free lozenges. Maintaining good oral hygiene is important to prevent infections.
Nausea and vomiting are common reactions to LuPSMA. These symptoms can be controlled with anti-nausea medications, which can be taken before treatment or as prescribed. Patients can also benefit from eating small, frequent meals, avoiding strong odors, and staying hydrated to alleviate nausea.
Anemia, a decrease in red blood cell count, can cause fatigue and pallor. To manage anemia, blood transfusions or medications that stimulate red blood cell production may be needed. Regular blood tests are crucial to monitor and adjust treatment as necessary.
Decreased appetite is another side effect that some patients may experience, which can affect overall health and energy levels. This can be managed by offering small, frequent meals and including high-calorie, high-protein foods in the diet. Consulting with a nutritionist can help develop a plan to maintain nutritional intake.
Constipation is another potential side effect. Increasing dietary fiber, ensuring adequate hydration, and using over-the-counter laxatives or stool softeners, as recommended by a healthcare provider, can help alleviate this issue.
Less Common but Notable Side Effects and Their Management
Myelosuppression, or bone marrow suppression, is a condition where the production of blood cells decreases, leading to an increased risk of infections, bleeding, or anemia. It requires careful monitoring with blood tests. In some cases, dose adjustments or temporary cessation of treatment may be required. Medications that stimulate bone marrow activity or transfusions may also be necessary.
Renal toxicity can impact kidney function, and it requires regular monitoring. Patients should stay well-hydrated before and after treatment. If kidney toxicity is detected, the treatment plan may need to be adjusted. Regular kidney function tests are essential to monitor for signs of kidney damage.
Electrolyte imbalances, such as changes in the levels of calcium, potassium, and sodium, can occur during treatment. These imbalances can cause symptoms like muscle cramps or irregular heart rhythms. Regular blood tests can help detect these issues, and appropriate supplementation can correct them.
Gastrointestinal issues such as abdominal pain or diarrhea may also occur. These can be managed by adjusting the diet, avoiding spicy or fatty foods, and using medications to treat diarrhea or manage abdominal pain, as directed by a healthcare provider.
Side Effects management
Patients receiving Pluvicto should undergo regular monitoring, including blood tests to assess for anemia, myelosuppression, and electrolyte imbalances, as well as kidney function tests. Open communication with the healthcare team is essential for managing side effects effectively and adjusting the treatment plan as needed.
By addressing side effects promptly and effectively, LuPSMA therapy can be better tolerated, allowing patients to continue treatment and achieve the best possible outcomes.

What is the Recommended Dosage of Pluvicto?
Pluvicto is available as an injection solution for IV use in a 1,000 MBq/mL (27 mCi/mL) single-dose vial.
For metastatic castration-resistant prostate cancer (mCRPC), Pluvicto is indicated for men with PSMA-positive mCRPC who have previously received androgen receptor pathway inhibitors (ARPI) and are suitable for delaying taxane chemotherapy or have already undergone taxane-based chemotherapy.
The recommended dosage is 7.4 GBq (200 mCi) administered intravenously every 6 weeks for up to 6 doses, or until disease progression or unacceptable toxicity occurs.
How is Pluvicto administered?
LuPSMA should be handled using aseptic technique and appropriate radiation shielding. The vial must be visually inspected for any particulates or discoloration before use, and it should be discarded if any issues are found. It should not be mixed with other IV solutions. The amount of radioactivity administered must be confirmed with a calibrated dose calibrator before and after administration. Any unused product must be disposed of according to local regulations.
The drug can be administered via IV injection with a syringe, gravity infusion, or peristaltic pump. In the case of reduced doses, the syringe method or an adjusted gravity infusion should be used. The IV catheter should be flushed with 10 mL of 0.9% NaCl before administration to ensure proper patency.
Pluvicto must be stored below 30ºC (86ºF) and protected from freezing. It should be kept in its original package with lead shielding to protect from ionizing radiation. Its shelf-life is 120 hours (5 days) from the time of calibration.
What to Avoid During Pluvicto Treatment?
Patients are advised to avoid radiation exposure, particularly to pregnant women and children, by using proper shielding when handling the drug.
Pluvicto should not be administered with other IV medications unless directed by a healthcare provider, and the IV catheter should be used exclusively for Pluvicto.
Activities that could increase the risk of infection or bleeding should be avoided, as the treatment may suppress bone marrow function. Patients should adhere to the prescribed dosage and schedule and consult a healthcare provider before skipping or delaying doses.
Pluvicto’s effectiveness over time
Pluvicto (lutetium Lu 177 vipivotide tetraxetan) has shown promising effectiveness in treating metastatic castration-resistant prostate cancer (mCRPC), particularly in patients previously treated with androgen receptor inhibitors or taxane-based chemotherapy. In the PSMAfore trial, Pluvicto resulted in a median radiographic progression-free survival (rPFS) of 9.3 months compared to 5.6 months with ARPI alone. Ongoing studies continue to evaluate its long-term impact, with early results suggesting it can delay disease progression and improve overall survival.
Ongoing trials with Pluvicto
Phase II Trial Evaluating 177Lu-PSMA-617 in Metastatic Clear Cell Renal Carcinoma (ccRCC) tests the efficacy and safety of 177Lu-PSMA-617 in metastatic ccRCC patients whose disease has progressed after prior treatments with immune checkpoint inhibitors and VEGFR-TKIs. The treatment aims to target PSMA-positive cancer cells with targeted radiation. Participants will receive up to six doses, with imaging and blood tests to monitor response. Key objectives include assessing disease control, progression-free survival, and overall survival.(NCT06783348)
Learn more about Kidney Cancer: Symptoms ,Causes, Stages, Diagnosis and Treatment on OncoDaily.
The CATCH-177 study (NCT06738303) compares Carboplatin and Cabazitaxel with 177Lu-PSMA-617 in metastatic castrate-resistant prostate cancer (mCRPC) that is resistant to hormone therapy and docetaxel. The goal is to identify the most effective treatment and optimal sequencing for patients with this aggressive form of prostate cancer.
The STARLiT trial (NCT06574880) aims to explore whether Pluvicto can reduce cancer recurrence and improve quality of life when used instead of androgen-deprivation therapy (ADT) alongside radiation therapy in patients with advanced local prostate cancer. Participants will receive one dose of Pluvicto, followed by radiation therapy after six weeks. A second dose of Pluvicto will be administered six weeks after radiation, with some participants receiving a third dose. This study evaluates whether Pluvicto can replace ADT to enhance treatment outcomes while minimizing side effects and improving patient compliance.
The study, titled “A Randomized, Multi-centre, Intra-patient Imaging and Dosimetry Crossover Study of Lutetium (177Lu) rhPSMA-10.1 and Lutetium (177Lu) Vipivotide Tetraxetan (Pluvicto®) in Patients with Non-curative Metastatic Prostate Cancer, NCT06516510) aims to compare the efficacy and dosimetry of 177Lu rhPSMA 10.1 and 177Lu vipivotide tetraxetan (Pluvicto®) in patients with metastatic prostate cancer.
Written by Mariam Khachatryan, MD
FAQ
What is Pluvicto?
Pluvicto is a treatment for metastatic prostate cancer. It delivers targeted radiation to cancer cells, helping to shrink or destroy tumors while minimizing damage to healthy tissue.
How does Pluvicto work to treat cancer?
Pluvicto targets prostate cancer cells by binding to a protein called PSMA on their surface. It then delivers radiation directly to the tumor, helping to eliminate cancer cells.
How is Pluvicto administered?
Pluvicto is administered through an IV infusion in a hospital or clinic. The process takes a short time, and patients may require multiple doses.
What are the common side effects of Pluvicto?
Common side effects include fatigue, nausea, dry mouth, and changes in blood counts. Some patients may also experience constipation or diarrhea.
Are there any serious side effects associated with Pluvicto?
Serious side effects can include kidney issues, bone marrow suppression, and allergic reactions. Regular blood tests help monitor these risks.


