THRIVE-131 trial, Pfizer’s latest late-stage study of inclacumab in people with sickle cell disease (SCD), has concluded without meeting its primary endpoint. The trial tested whether the investigational P-selectin inhibitor could significantly reduce the rate of vaso-occlusive crises (VOCs) compared with placebo over 48 weeks. While the therapy was generally well tolerated, Pfizer announced that the results fell short of expectations, underscoring the challenges of developing effective new treatments for this lifelong inherited blood disorder.

“We recognize this news is disappointing for the sickle cell community, and we share their disappointment.While the THRIVE-131 results did not meet our expectations, we remain committed to better understanding these results and sharing them with the medical and sickle cell community in the interest of advancing our collective understanding of sickle cell disease. We remain focused on our mission of bringing much-needed treatments to patients with sickle cell disease. We are deeply grateful to the participants and investigators for their contributions to this important work. Their efforts are invaluable in informing future sickle cell research.”
said Michael Vincent, M.D., Ph.D., Chief Inflammation & Immunology Officer, Pfizer.
Inclacumab: Drug Profile and Mechanism of Action
Inclacumab is an investigational monoclonal antibody that targets P-selectin, a cell adhesion molecule involved in sickle cell disease. In SCD, P-selectin drives the binding of sickled red blood cells and platelets to blood vessels, leading to inflammation and vaso-occlusive crises (VOCs).
By blocking P-selectin, inclacumab is designed to reduce this abnormal adhesion and lower the risk of VOCs. It is given as an intravenous infusion every 12 weeks, offering a potentially convenient dosing schedule.
What is Sickle Cell Disease?
Sickle cell disease (SCD) is a group of inherited blood disorders caused by mutations in the HBB gene, which encodes the beta chain of hemoglobin. This leads to the production of abnormal hemoglobin S (HbS). When oxygen levels drop, HbS polymerizes, causing red blood cells to change shape from their normal round, flexible form into rigid, crescent- or “sickle”-shaped cells.
How Sickle Cells Affect the Body
- Shortened lifespan of red blood cells: Normal RBCs live ~120 days, but sickled cells survive only 10–20 days, leading to chronic hemolytic anemia.
- Vaso-occlusion: The rigid, sticky sickle cells block small blood vessels, causing painful episodes called vaso-occlusive crises (VOCs) and damaging organs over time.
- Chronic inflammation: Endothelial injury and abnormal adhesion contribute to vascular inflammation and complications.
Clinical Manifestations of Sickle Cell Disease
The clinical manifestations of sickle cell disease are diverse and can affect nearly every organ system, beginning early in life and continuing throughout adulthood. One of the hallmarks of the disease is chronic hemolytic anemia, which typically presents with fatigue, pallor, and delayed growth and development in children. This anemia results from the markedly shortened lifespan of sickled red blood cells, which survive only 10 to 20 days compared to the 120 days of normal erythrocytes.A defining feature of the condition is the occurrence of vaso-occlusive crises, often referred to as pain crises.
These episodes are characterized by sudden, severe pain involving the bones, chest, or abdomen and are caused by sickled red blood cells obstructing the microvasculature. Such crises are unpredictable, recurrent, and represent a major cause of hospitalizations and reduced quality of life in patients.
Another life-threatening complication is acute chest syndrome, which presents with respiratory distress, fever, and pulmonary infiltrates on imaging. This condition is caused by vaso-occlusion and inflammation in the pulmonary vasculature and is a leading cause of mortality in both pediatric and adult patients.
Sickle cell disease also increases the risk of cerebrovascular complications, including ischemic and hemorrhagic strokes. Children are particularly vulnerable, with stroke risk peaking between the ages of two and 16, although adults remain at elevated risk throughout life.
In addition to acute crises, progressive organ damage is a frequent long-term outcome of the disease. The kidneys may develop chronic kidney disease, the spleen often becomes nonfunctional due to repeated infarctions, and other organs such as the liver, eyes, and bones are also at risk of chronic injury. The cumulative effect of these complications contributes significantly to morbidity and mortality.
Finally, the loss of splenic function, known as functional asplenia, greatly increases susceptibility to life-threatening infections, particularly from encapsulated bacteria. This predisposition underscores the importance of preventive measures such as vaccinations and prophylactic antibiotics in pediatric care.
Current Treatment Approaches
Treatment of sickle cell disease relies on a combination of supportive, targeted, and potentially curative strategies.
Supportive Care
- Blood transfusions (to prevent complications like stroke).
- Pain management during VOCs.
- Hydroxyurea, which increases fetal hemoglobin (HbF) and reduces sickling.
Targeted Therapies
- Crizanlizumab (P-selectin inhibitor, approved 2019) – reduces VOC frequency.
- Voxelotor (OXBRYTA®) – targeted hemoglobin S polymerization inhibitor (withdrawn in 2024 pending safety re-evaluation).
- L-glutamine – reduces oxidative stress and VOC frequency.
Curative Options
- Hematopoietic stem cell transplantation (HSCT) – curative in some patients, though limited by donor availability and risks.
- Gene therapy & gene editing (e.g., exagamglogene autotemcel [exa-cel], approved in 2023 in some regions) – emerging curative strategies.
About THRIVE-131 trial
The Phase 3 THRIVE-131 trial was a global, randomized, double-blind, placebo-controlled study designed to evaluate the efficacy and safety of inclacumab in sickle cell disease. The trial enrolled 241 participants aged 16 years and older who had experienced between two and ten vaso-occlusive crises in the previous year. Patients were randomized to receive intravenous inclacumab or placebo every 12 weeks over a 48-week treatment period. The primary endpoint was the reduction in the rate of vaso-occlusive crises during the study. Participants completing THRIVE-131 were eligible to continue into THRIVE-133 OLE, an open-label extension study designed to assess the long-term safety of inclacumab.
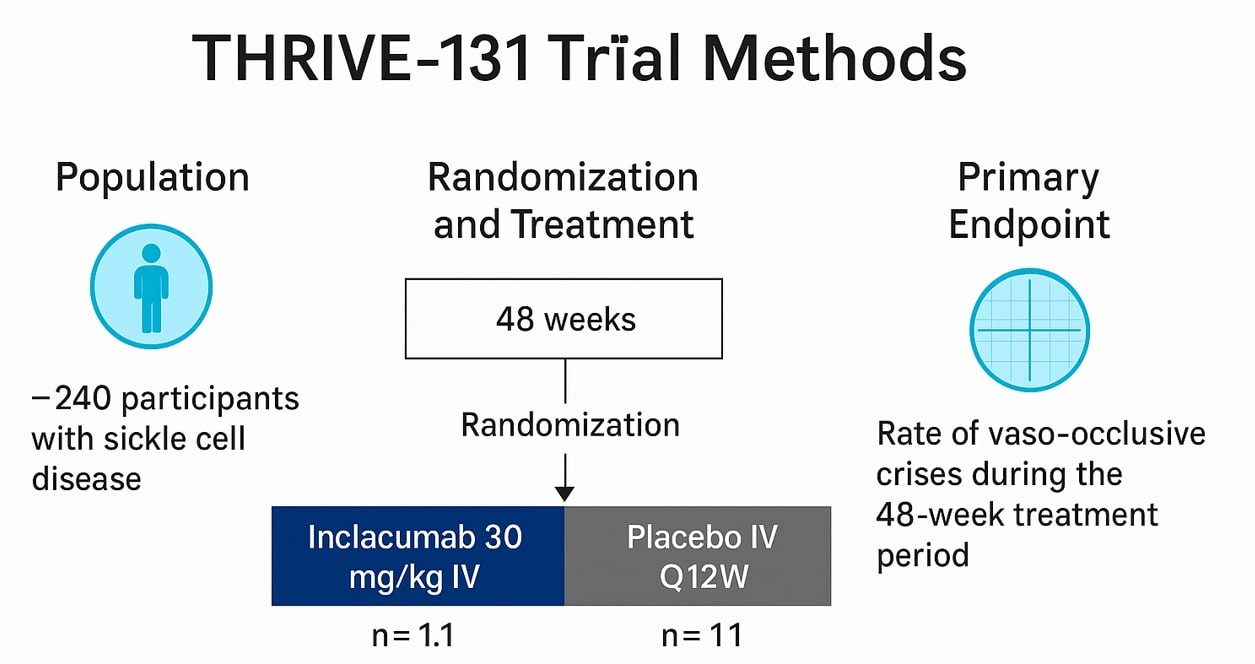
Key Results
The study did not meet its primary endpoint, as inclacumab failed to demonstrate a significant reduction in the rate of vaso-occlusive crises compared with placebo. Despite this, the treatment was generally well tolerated. The most commonly reported treatment-emergent adverse events, observed in both the inclacumab and placebo arms, were anemia, arthralgia, back pain, headache, malaria, sickle cell crisis, and upper respiratory tract infection.
Even though the results of the THRIVE-131 trial did not meet its primary endpoint, Pfizer remains committed to the sickle cell community and is actively advancing the next steps for its broader SCD portfolio.
Other Drugs Developed by Pfizer for SCD
Pfizer has also worked on another therapy for sickle cell disease, OXBRYTA® (voxelotor), an oral drug developed to inhibit hemoglobin S polymerization and reduce red blood cell sickling. OXBRYTA received accelerated approval from the FDA in 2019 and was subsequently approved in more than 35 countries.
However, in September 2024, Pfizer voluntarily withdrew the drug from all global markets and discontinued its clinical trials after the totality of data indicated that the benefit no longer outweighed the risks, with evidence of increased vaso-occlusive crises and fatal events. Despite this setback, Pfizer underscored its commitment to patient safety and announced plans to publish a comprehensive analysis of OXBRYTA data to inform future advances in sickle cell disease therapy.
Taken together, Pfizer’s programs with inclacumab and OXBRYTA reflect both the challenges of advancing therapies in sickle cell disease and the company’s determination to continue seeking better treatment options for patients worldwide.
Read more information here


