On October 29, 2024, the FDA granted accelerated approval for asciminib (Scemblix, Novartis AG) to treat adult patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP).
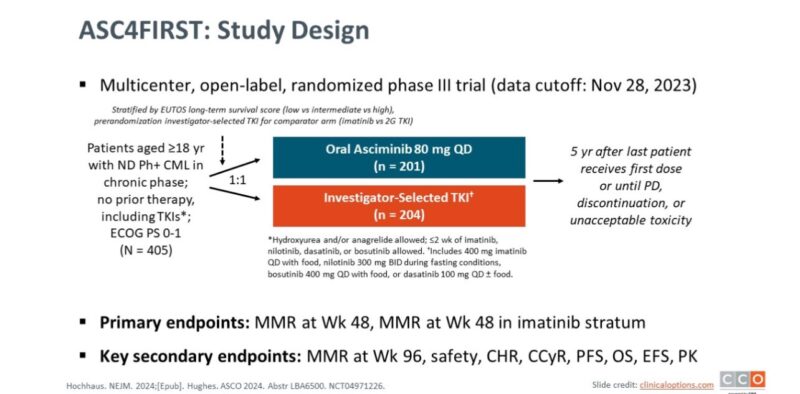
This approval is based on findings from the ASC4FIRST trial (NCT04971226), a multicenter, randomized study comparing asciminib to investigator-selected tyrosine kinase inhibitors (TKIs).
Primary investigator of the trial:
Andreas Hochhaus
Andreas Hochhaus, MD, is a Professor of Internal Medicine, Hematology, and Oncology and the interim Head of the Department of Hematology and Medical Oncology at University Medical Center Jena in Germany.
He specializes in optimizing treatments for chronic myelogenous leukemia (CML) and has been a longstanding contributor to the German CML Study Group’s randomized CML Studies I-IV. His research focuses on molecular monitoring of minimal residual disease, mechanisms of resistance in CML, and targeted therapies for various cancers.

Efficacy
The ASC4FIRST trial enrolled 405 patients, who were randomized in a 1:1 ratio to receive either asciminib or standard TKIs, including imatinib, nilotinib, dasatinib, or bosutinib. The primary efficacy endpoint was the major molecular response (MMR) rate at 48 weeks. Results showed that:
The MMR rate for asciminib was 68% (95% CI: 61-74) compared to 49% (95% CI: 42-56) for the TKI group, representing a statistically significant difference of 19% (p < 0.001).
In the imatinib stratum specifically, the MMR rate was 69% for asciminib versus 40% for TKIs, with a difference of 30% (p < 0.001).
Further analysis indicated that asciminib also led to higher rates of deep molecular responses, with MR4 rates at week 48 being 38.8% for asciminib versus 20.6% for TKIs, and MR4.5 rates of 16.9% versus 8.8%, respectively.
Safety Profile
In terms of safety, the most common adverse reactions reported in at least 20% of patients included:
- Musculoskeletal pain
- Rash
- Fatigue
- Upper respiratory tract infection
- Headache
- Abdominal pain
- Diarrhea
Laboratory abnormalities seen in over 40% of patients included decreased lymphocyte count, leukocyte count, platelet count, neutrophil count, and corrected calcium levels.
Among patients treated with asciminib:
- Grade 3 or higher adverse effects occurred in 38%, compared to higher rates in those treated with imatinib (44.4%) and second-generation TKIs (54.9%).
- Discontinuation due to adverse effects was lower with asciminib at 4.5%, compared to 11.1% for imatinib and 9.8% for second-generation TKIs.

Dosage and Administration
The recommended dosage of asciminib is either:
- 80 mg orally once daily, or
- 40 mg orally twice daily, taken approximately every 12 hours.
Conclusion
Asciminib’s approval marks a significant advancement in treating newly diagnosed Ph+ CML in CP, offering improved efficacy and a favorable safety profile compared to existing TKIs. As noted by experts in the field, this therapy has the potential to change treatment paradigms for CML patients by providing an effective first-line option that minimizes side effects while maximizing therapeutic outcomes.
On this occasion, Oncology Brothers shared a post on X:
“Asciminib now (accelerated) US FDA approved for Ph+ CML based off ASC4First.
80mg/QDay or 40mg Q12Hrs
- Major molecular response at 48weeks 68% (vs 49%)
- AEs: Myalgia, rash, URI, marrow suppression, toxicity
- Is this now new SoC?
Even though we have deeper MMR early on, we need long term/mature data to even extrapolate if this can be stopped and offer this as time limited therapy if MMR is achieved. Another option (different AEs) but majority will still likely get Imatinib upfront on my end.”
For more posts like this, visit oncodaily.com.


