Gastric cancer remains one of the most common and lethal cancers worldwide, with China carrying a significant share of the global burden. Standard chemotherapy provides limited benefit, with median survival rarely exceeding one year. The advent of immune checkpoint inhibitors, such as nivolumab and pembrolizumab, has expanded treatment options, especially for patients with PD-L1–positive tumors.
In this context, Sugemalimab plus Chemotherapy has emerged as a promising first-line option. However, questions remain regarding its economic value. To address this, researchers in China conducted a cost-effectiveness analysis of Sugemalimab plus Chemotherapy versus chemotherapy alone, using data from the Phase III GEMSTONE-303 trial.
Study Design
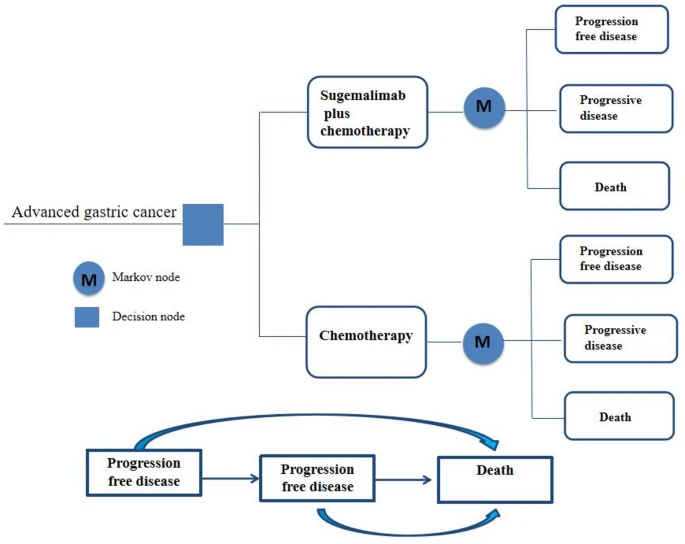
Patients with unresectable, locally advanced, or metastatic gastric adenocarcinoma were included, focusing particularly on those with PD-L1 combined positive scores (CPS) of 5 or higher. The model incorporated three health states—progression-free survival, disease progression, and death—and simulated outcomes over a 10-year horizon from the perspective of the Chinese healthcare system.
Direct medical costs (drug acquisition, adverse event management, supportive care) were estimated using Chinese healthcare data, while quality-adjusted life years (QALYs) were used as the measure of effectiveness. The willingness-to-pay threshold was set at approximately USD 40,344 per QALY, reflecting three times the Chinese GDP per capita.
Clinical Background: GEMSTONE-303 Trial
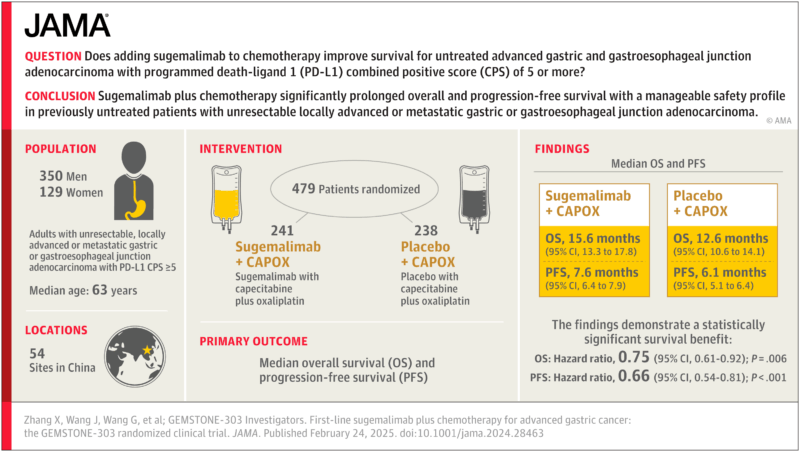
The clinical foundation for this economic analysis comes from the GEMSTONE-303 Phase III trial, a randomized, double-blind, placebo-controlled study conducted across 54 sites in China. Results were published in JAMA (February 24, 2025; 333:1305–1314. doi:10.1001/jama.2024.28463). In this trial, 479 patients with previously untreated, unresectable locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma and a PD-L1 CPS of 5 or higher were enrolled.
Treatment with sugemalimab plus CAPOX chemotherapy significantly prolonged survival compared with chemotherapy alone, with median overall survival of 15.6 months versus 12.6 months, and median progression-free survival of 7.6 months versus 6.1 months. These benefits were achieved with a manageable safety profile, making GEMSTONE-303 the first study to demonstrate a statistically significant survival advantage for an anti–PD-L1 antibody combined with chemotherapy in this disease.
Results
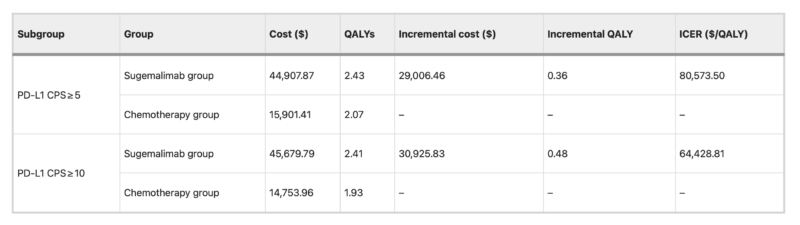
In patients with PD-L1 CPS ≥5, the total cost of sugemalimab plus chemotherapy reached USD 44,907.87 compared with USD 15,901.41 for chemotherapy alone. This produced an incremental gain of 0.36 QALYs at an added cost of USD 29,006.46, resulting in an ICER of USD 80,573.50 per QALY. For those with CPS ≥10, the sugemalimab regimen cost USD 45,679.79 versus USD 14,753.96 for chemotherapy, with a 0.48 QALY gain and an ICER of USD 64,428.81 per QALY. Both values were well above the willingness-to-pay threshold of USD 40,344.
Sensitivity analyses showed the ICER was most influenced by drug price and utility values for progression-free and progressive disease, but results remained above threshold across all variations. Probabilistic sensitivity analysis confirmed a 0% probability of cost-effectiveness in CPS ≥5 and only 1.5% in CPS ≥10. Notably, a 40% price reduction lowered the ICER in CPS ≥10 to USD 41,000.04 per QALY, raising the probability of cost-effectiveness to 48%. The findings were recently published in Scientific Reports (Nature Portfolio).
What is Sugemalimab and How does it work?
Sugemalimab is a fully human monoclonal antibody that belongs to the class of immune checkpoint inhibitors. Developed by CStone Pharmaceuticals in collaboration with EQRx, it is designed to target programmed death-ligand 1 (PD-L1), a protein frequently expressed on the surface of cancer cells. By blocking PD-L1, Sugemalimab helps the body’s immune system recognize and attack tumor cells.
Under normal circumstances, PD-L1 binds to PD-1 receptors on T cells, effectively switching off the immune response and allowing cancer cells to escape detection. Sugemalimab prevents this binding, restoring T-cell activity and boosting the body’s natural ability to fight cancer. This mechanism makes it particularly valuable in gastric cancer, gastroesophageal junction cancer, lung cancer, and lymphoma, where PD-L1 expression contributes to immune evasion.
Conclusion
While the GEMSTONE-303 trial published in JAMA confirmed that sugemalimab plus chemotherapy significantly improves overall and progression-free survival with a manageable safety profile, the subsequent Scientific Reports cost-effectiveness analysis concluded that this regimen is not currently cost-effective in China compared with chemotherapy alone. However, drug price reductions and biomarker-driven patient selection could improve its economic viability, highlighting the ongoing need to balance clinical innovation with healthcare sustainability.