Colorectal liver metastases (CLM) remain a defining challenge in gastrointestinal oncology, where surgical resection offers the only potential for long-term survival. Advances in systemic therapy have broadened the pool of patients eligible for curative surgery, allowing many previously unresectable cases to be downstaged to operable disease. Among preoperative chemotherapy options, oxaliplatin- and irinotecan-based regimens play a pivotal role, yet the ideal treatment intensity before surgery—and its implications for operative safety and long-term outcomes—are still debated.
This article explores how a large international collaboration set out to clarify that question by comparing triplet (FOLFOXIRI) versus doublet (FOLFOX/FOLFIRI) preoperative chemotherapy in patients undergoing curative liver resection for colorectal liver metastases.
Publication Details
The study, published on October 1, 2025, in the Annals of Surgical Oncology (Open Access, Hepatobiliary Tumors section), investigates how different chemotherapy intensities influence perioperative safety and long-term outcomes in patients with resectable colorectal liver metastases. It provides one of the most comprehensive analyses to date on the role of FOLFOXIRI as a preoperative regimen, comparing it directly with standard doublet therapy across multiple international centers.
Trial Design and Endpoints
This retrospective analysis included 1,711 patients who underwent first curative-intent hepatectomy after preoperative FOLFOXIRI or FOLFOX/FOLFIRI.
- FOLFOXIRI group: 160 patients (9.4%)
- Doublet group: 1,551 patients (90.6%)
Patients receiving multiple chemotherapy lines were excluded. Primary endpoints included perioperative morbidity, 90-day mortality, and overall survival (OS). Secondary endpoints evaluated predictors of postoperative complications and long-term survival. Ethical approval was obtained from all participating institutions, and data collection followed the Declaration of Helsinki.
Key Results
Patients receiving FOLFOXIRI were younger (median 55 vs. 59 years, p < 0.001), had more synchronous metastases (93.7% vs. 78.7%, p < 0.001), and a higher median tumor burden score (TBS 5.6 vs. 4.1, p < 0.001).
Both groups received a median of six chemotherapy cycles.
Intraoperative and Early Outcomes
- Major hepatectomy: 44.4% (triplet) vs. 35.3% (doublet), p = 0.024
- Estimated blood loss: 300 mL vs. 200 mL, p = 0.006
- Intraoperative transfusion: 8.1% vs. 4.4%, p = 0.034
- 90-day mortality: 1.2% vs. 0.6%, ns
- Major complications (Clavien–Dindo ≥3): 13.3% vs. 10.0%, ns
While bile leak was more common in the triplet group (10.0% vs. 4.6%, p = 0.003), rates of post-hepatectomy liver failure were comparable. Independent predictors of major morbidity included >6 chemotherapy cycles, simultaneous colorectal resection, major hepatectomy, and operative time >300 minutes.
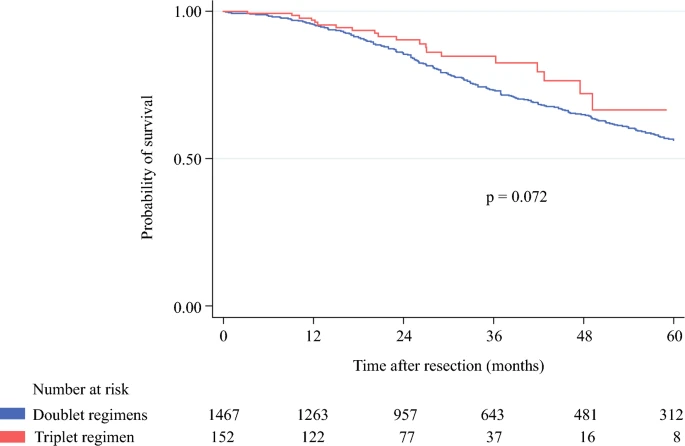
Long-Term Survival
1-, 3-, and 5-year OS:
- FOLFOXIRI: 97.0%, 84.7%, 66.6%
- Doublet: 95.4%, 73.3%, 56.4% (p = 0.072)
- Median OS: 75.8 months (doublet) vs. not reached (triplet)
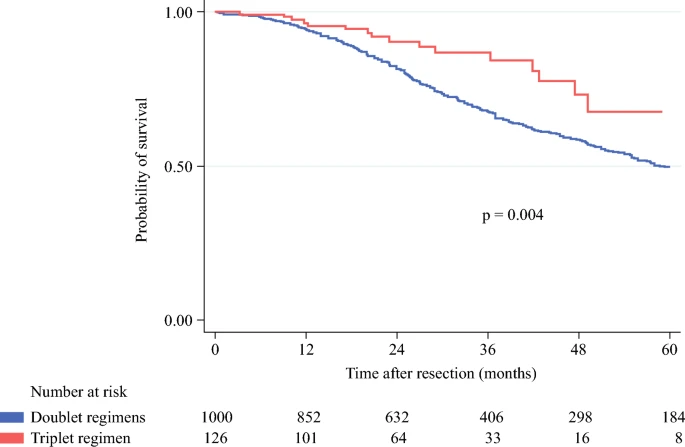
In multivariable analysis, medium–high TBS, extrahepatic disease, positive resection margin, and RAS mutation were independent predictors of poor OS.
Importantly, in the medium–high TBS subgroup, triplet chemotherapy conferred a significant survival benefit:
- 5-year OS: 67.6% (FOLFOXIRI) vs. 50.1% (FOLFOX/FOLFIRI), p = 0.004
- FOLFOXIRI remained an independent predictor of improved survival (p = 0.038).
Discussion
Although FOLFOXIRI was more frequently administered to patients with biologically aggressive disease and greater tumor burden, it did not increase operative mortality or major complication rates compared with doublet therapy. The study therefore supports the safety of triplet chemotherapy before hepatectomy and suggests that patients with higher tumor burden may derive a survival advantage.
The authors underscore that extending preoperative treatment beyond six cycles increases postoperative risk, reinforcing the importance of proceeding to surgery promptly once resectability is achieved. They also note the growing adoption of parenchyma-sparing liver resections, which preserve functional tissue while maintaining oncologic completeness.
Conclusion
This large, multicenter investigation—the first to directly compare triplet and doublet preoperative regimens in resectable colorectal liver metastases—demonstrates that FOLFOXIRI maintains surgical safety and may improve long-term outcomes in patients with high disease burden. For oncology teams managing advanced metastatic colorectal cancer, these findings support a tailored, multidisciplinary strategy that integrates triplet chemotherapy as an effective neoadjuvant option followed by timely curative resection.

Read about the 10 Must-Read Posts in GI Oncology from the first week of September on OncoDaily.