Bristol Myers Squibb (BMS) and Janux Therapeutics have formed a partnership to co-develop a novel tumor-activated T cell engager therapy for solid tumors. Under the deal, Janux will apply its proprietary technology through late-preclinical development and IND (Investigational New Drug) submission, after which BMS will lead clinical trials and global commercialization. Janux will remain involved through the first Phase 1 study, supporting BMS during early clinical evaluation.
“This collaboration marks a significant milestone for Janux, validating the strength of our tumor-activated platforms and expanding our reach in solid tumor oncology, by combining Janux’s innovative technology with Bristol Myers Squibb’s deep expertise in clinical development and global commercialization, we aim to accelerate the delivery of transformative therapies to patients with difficult-to-treat cancers.”

David Campbell, Ph.D./januxrx.com
Scientific Rationale: T Cell Engagers in Solid Tumors
T cell engagers (TCEs) are bispecific antibody-based drugs that bind cancer cells on one end and T cells on the other, physically bringing immune cells into contact with tumors to trigger cell killing. This approach has proven effective in certain blood cancers – for example, CD3×CD19 bispecifics like blinatumomab in leukemia – but translating TCEs to solid tumors has been challenging. Traditional T cell engagers can latch onto healthy tissues that share the target antigen or over-activate immune cells systemically, leading to serious toxicities such as cytokine release syndrome (CRS). They also tend to have short half-lives, requiring continuous infusion or frequent dosing. These issues have forced dose limitations and contributed to disappointing results for many solid tumor TCE programs.
Janux’s technology directly addresses these limitations through a “tumor-activated” or “masked” TCE design. Each Janux TCE (branded a TRACTr) is equipped with peptide “masks” that cover the T cell-binding domains (e.g. the anti-CD3 region) while the drug circulates in the bloodstream. These masks are attached by linkers that can be cleaved by proteases uniquely active in the tumor microenvironment. In essence, the engager remains inert, unable to bind T cells or activate them, until it reaches the tumor site, where tumor-specific enzymes cut off the masks. At that point the drug is “unmasked” locally, regaining full bispecific binding activity against the tumor cells and T cells.
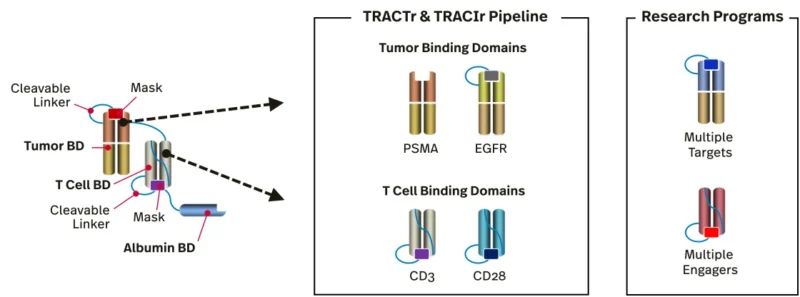
Janux also integrates an albumin-binding domain into the molecule to extend the drug’s half-life, and this too is removed by tumor-localized proteases to ensure that once activated, the TCE does not persist long outside the tumor. The net result is a precision immunotherapy that should preferentially act on tumor tissue while sparing normal cells, thereby widening the therapeutic window in solid cancers.
Preclinical studies have provided proof-of-concept for Janux’s masked TCE approach. For example, a Janux TCE targeting EGFR (epidermal growth factor receptor) with dual peptide masks demonstrated over 300× reduction in EGFR binding and 1,000× reduction in CD3 binding when masked (DiRaimondo et al., 2022).
In tumor models, the masked EGFR×CD3 TCE remained inactive until protease activation, after which it showed potent T cell-mediated killing comparable to an unmasked TCE. Crucially, in non-human primate studies, the tumor-activated EGFR TCE achieved much higher drug exposure with far less toxicity: monkeys tolerated doses yielding >3,000-fold higher peak drug levels than an unmasked EGFR bispecific, with markedly lower cytokine release and negligible healthy-tissue damage. These data suggest Janux’s platform can mitigate CRS and off-tumor toxicity while improving pharmacokinetics, a profile that could overcome the roadblocks that plagued earlier solid tumor TCEs.
The New BMS-Janux Therapeutic Candidate
Under the BMS collaboration, Janux will engineer a novel T cell-engaging therapeutic against an undisclosed solid tumor antigen that is known to be expressed in several cancer types. While Janux and BMS have not revealed the target’s identity, it is described as a “validated” antigen which suggests it’s a well-established cancer marker that has been clinically pursued by others. What’s clear is that the candidate will leverage Janux’s TRACTr/TRACIr platform, meaning it will be a bispecific molecule with Janux’s tumor-only activation features.
Importantly, the “tumor-activated” design is expected to enable higher dosing or more potent T cell activation than an unmasked bispecific could achieve, since off-tumor effects are minimized. This could translate into stronger tumor responses without excessive immune-related side effects. Such a result would distinguish the BMS/Janux drug in a competitive field.
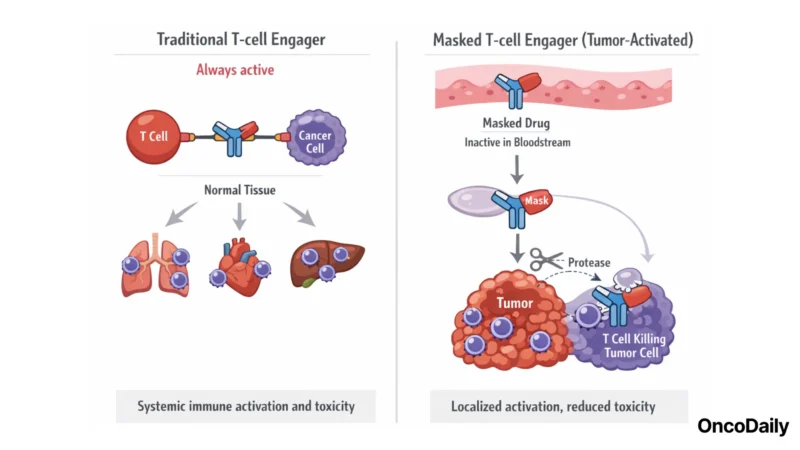
Traditional T-cell engagers activate T cells throughout the body, while tumor-activated (masked) engagers stay “off” in circulation and switch “on” mainly inside the tumor—helping reduce systemic toxicity.
Impact on the Oncology Treatment Landscape
The BMS-Janux alliance is significant on multiple levels. First, it reflects a broader evolution in cancer immunotherapy: the move toward conditionally activated, highly targeted treatments. Checkpoint inhibitors taught us that unleashing T cells can control tumors, but also that specificity matters to avoid collateral damage. T cell engagers are a potent way to direct T cells, and Janux’s platform adds a crucial layer of control (spatial activation in tumors only).
If successful, this approach could overcome the historical toxicity bottleneck and finally make TCEs viable for solid tumors, potentially benefiting patients with cancers like prostate, lung, colorectal, and others that overexpress the chosen targets. In essence, it could expand the T cell redirection strategy beyond hematologic malignancies into mainstream solid tumor oncology, filling a gap in the current treatment landscape.

Read About Cancer Immunotherapy on OncoDaily.
From a pharmaceutical development perspective, the partnership underlines the value of innovative biotech platforms to big pharma pipelines. BMS gains access to Janux’s cutting-edge TRACTr technology without having to reinvent it in-house, while Janux gains a powerful partner to accelerate clinical development. BMS’s involvement should expedite the progression of the unnamed program through trials, given BMS’s experience in running large oncology studies and navigating regulatory pathways. This is important because speed and scale will likely be needed to compete.
Other companies are also advancing bispecific T cell engagers for solid tumors. BMS entering the fray with Janux’s solution signals that the race to bring bispecific immunotherapies for solid tumors to market is intensifying. Notably, no T cell engager for a solid tumor is approved yet (as of early 2026), so whichever partnerships or programs succeed could define a new standard of care.
By targeting a “validated antigen” and leveraging positive proof-of-concept data from Janux’s existing programs, BMS and Janux are de-risking the endeavor as much as possible. Early human data from JANX007 and JANX008 provides confidence that the platform works; the new program will build on that foundation. For the oncology community, this collaboration is a vote of confidence in tumor-activated therapies as a path to “transformative therapies for difficult-to-treat cancers”. It exemplifies how companies are responding to the challenge of solid tumors by designing smarter, more selective immune-based drugs.
In the broader biotech context, this partnership highlights a trend: major oncology companies are actively scouting for technologies to make bispecific T cell engagers safer and more effective. There is a recognition that while TCEs have demonstrated remarkable efficacy in certain settings, innovation is needed to conquer solid tumors. Janux’s tumor-activated strategy is one of the promising solutions being tested. If it succeeds, it could pave the way for a new class of precision immunotherapies that deliver potent T cell attacks to tumors without the typical systemic toxicity. Such therapies might significantly improve outcomes in cancers that are currently hard to treat, and could be used either alone or in combination with other treatments.
Overall, the BMS–Janux alliance is a science-driven partnership focused on addressing a key challenge in immuno-oncology. Much work remains, the therapeutic must prove itself in preclinical studies and then in clinical trials. But the scientific rationale is strong, and both companies are bringing complementary strengths to the endeavor. As Janux continues its ongoing Phase 1 trials (for JANX007 and JANX008) and advances this new partnered program, the oncology community will be watching closely. If tumor-activated T cell engagers live up to their promise, they could become a cornerstone of future cancer treatment, offering new hope for patients with difficult-to-treat solid tumors.
Written by: Semiramida Nina Markosyan, Editor, OncoDaily Canada


