Becton Dickinson (BD) has received U.S. FDA 510(k) clearance for its new EnCor EnCompass™ Breast Biopsy and Tissue Removal System, a state-of-the-art, multi‑modality vacuum-assisted biopsy device. This next-generation system enables minimally invasive breast tissue biopsy across all major imaging platforms – including stereotactic (x-ray), ultrasound, and MRI guidance, using a single integrated console. The clearance paves the way for BD to launch the EnCor EnCompass system in early 2026, expanding the company’s breast health portfolio and providing clinicians with a versatile new tool in breast cancer diagnosis and care.

You Can Read More About Breast Cancer Diagnosis and Care on OncoDaily.
Advancing Minimally Invasive Breast Biopsy
Image-guided needle biopsy has largely replaced open surgical biopsy as the standard of care for diagnosing breast lesions. Over the past two decades, radiologists have increasingly turned to percutaneous biopsy techniques, guided by mammography, ultrasound or MRI, because they offer accuracy comparable to surgery with far less morbidity. Extensive data confirm that large-core needle biopsies (including vacuum-assisted devices) can achieve diagnostic yields on par with excisional biopsy, while dramatically reducing procedure time, cost, scarring, and complication rates. As a result, most suspicious breast findings (e.g. masses, microcalcifications, areas of distortion) are now evaluated initially with image-guided core needle or vacuum-assisted biopsies, reserving surgery for confirmed malignancies or lesions not amenable to percutaneous sampling.
Vacuum-assisted breast biopsy (VABB) systems were introduced in the mid-1990s (first with the Mammotome® device) and represented a major innovation in minimally invasive breast care. Unlike standard spring-loaded core needles that retrieve a single small sample (~15 mg of tissue per core) , vacuum-assisted devices use suction and a rotating cutting cannula to acquire larger, contiguous tissue samples – often obtaining 6–12 specimens with one probe insertion. This yields substantially more tissue (an 11-gauge vacuum probe typically captures ~100 mg per sample vs ~15 mg with 14G core needle ) and can reduce the risk of under-sampling a lesion (Nakano et al., 2018).
In fact, lab studies have shown that a 7-gauge vacuum probe can extract up to 0.36 g of tissue in a single pass, the largest among modern devices (Nakano et al., 2018). By acquiring ample tissue, VABB improves diagnostic confidence for conditions like atypical ductal hyperplasia or early ductal carcinoma in situ (DCIS), which can be missed or understaged with small core samples. As Dr. Satoko Nakano and colleagues noted in a 2018 review, while traditional core needle biopsy is less invasive, “VAB uses a suction system, which allows a larger tissue sample to be obtained from a single insertion” often enabling more accurate histopathology and reducing the need for repeat biopsies.
Crucially, vacuum-assisted systems also opened the door to percutaneous removal of benign or high-risk breast lesions. Over time, VABB evolved beyond diagnosis into vacuum-assisted excision (VAE) for small lesions that would otherwise require surgery. Many studies have reported complete lesion excision in ~72–99% of cases using VAE for lesions under ~3 cm (Zouzos et al., 2025). For example, ultrasound-guided vacuum excision is now an accepted alternative to surgery for fibroadenomas and other benign tumors. A recent single-center study of 163 fibroadenomas (average ~2.4 cm) found that 98.8% of lesions were completely removed with vacuum excision, with only 2 cases (1.2%) of incomplete excision (Rupa & Kushvaha, 2021).
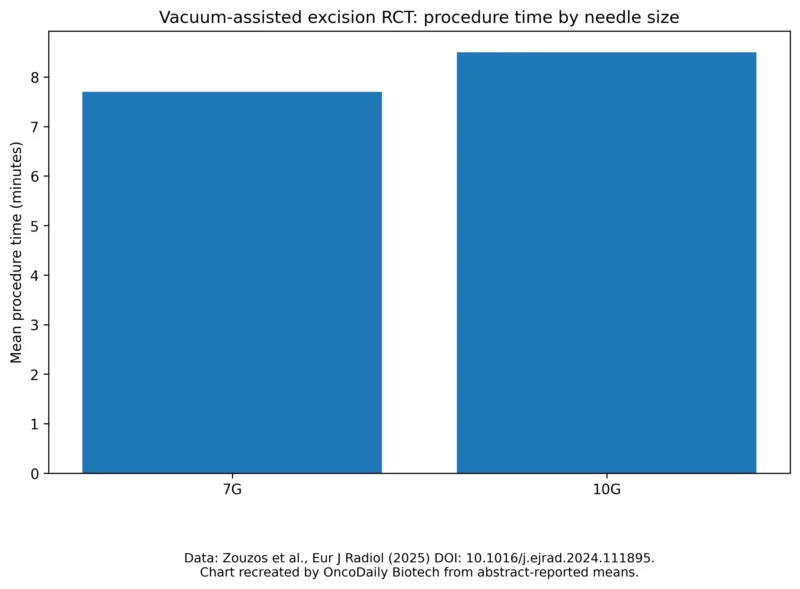
Mean procedure time in randomized trial of 7G vs 10G VAE.
Zouzos et al., randomized trial (Eur J Radiol).
No patients required stitches, significant scars were avoided, and complications were minimal – about 3% experienced mild hematoma and none had infections (Rupa & Kushvaha, 2021). “US-guided VAE of benign breast lesions offers a safe, effective…alternative to open surgery” for appropriate patients, the authors concluded. Likewise in Europe, vacuum excision is increasingly used to manage B3 lesions (lesions of uncertain malignant potential such as atypias or papillomas) after diagnostic biopsy (Zouzos et al., 2025), in order to remove the lesion completely without immediate surgery. However, experts caution that if malignancy is later detected in a vacuum-excised lesion, standard surgical re-excision is still required to ensure clear margins (Brzuszkiewicz et al., 2022).
The EnCor EnCompass™ System: Multi‑Modality and User-Focused Design
BD’s new EnCor EnCompass system builds upon this vacuum-assisted biopsy legacy with technological enhancements aimed at flexibility and efficiency. The system consists of a console and a lightweight handheld biopsy probe (driver) that can be used under all three imaging modalities – X-ray (stereotactic mammography), ultrasound, and MRI – with the same device. This unified approach addresses a long-standing need.
www.bd.com
Key features of the EnCor EnCompass™ include several innovations aimed at improving control over the biopsy procedure :
-
360° Rotating Vacuum Aperture: Samples all quadrants without reinserting the needle.
-
Adjustable Vacuum and Notch Size: Real-time control of suction and sample size; notch up to 20 mm (10G/12G) or 30 mm (7G) for larger cores or excisions.
-
Multiple Gauge Options (12G, 10G, 7G): Includes large 7G option for higher tissue yield and fewer repeat biopsies.
-
Enhanced Imaging Visibility: Echogenic cannula and illuminated specimen chamber for immediate sample verification.
-
Ergonomics and Workflow: Wireless foot pedal, touchscreen console, automated insertion, and on-probe marker deployment.
“The EnCor EnCompass™ Biopsy System combines multi-modality capability and enhanced control into one platform that supports intraprocedural customization and helps streamline the biopsy process.”

Dr. Shadi Aminololama-Shakeri/boonelab.bme.ucdavis.edu
Dr. Aminololama-Shakeri’s comments underscore how the device’s flexibility, from imaging modality to adjustable sampling, may improve the biopsy experience for both clinicians and patients. By enabling “intraprocedural customization”, radiologists can respond to what they see during the biopsy (such as adjusting suction or sample size if a lesion is not yielding enough tissue), potentially improving tissue yield and procedural efficiency.
Clinical Context: Indications and Performance
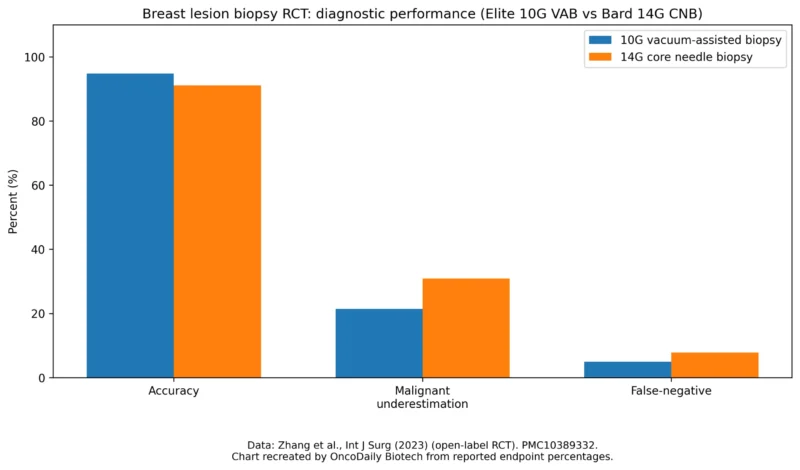
Randomized trial endpoint rates: 10G VAB vs 14G CNB in ultrasound-visible breast lesions.
Source: Zhang et al. (open-label RCT), endpoint percentages reported in the paper.
According to BD, the EnCor EnCompass is indicated “to acquire breast tissue for histologic examination with partial or complete removal of the abnormality”. In practice, this means the system can be used for both diagnostic biopsies (sampling a lesion to determine if it is cancer) and for therapeutic excision of certain lesions during the same minimally invasive procedure. Common indications include:
-
Suspicious imaging findings (BI-RADS 4 or 5): for example, clusters of microcalcifications seen on mammography (typically biopsied with stereotactic guidance), or small masses and architectural distortions found on ultrasound or MRI. In these cases, EnCor EnCompass would be used to retrieve multiple core samples to establish a diagnosis. Clinical studies have shown that percutaneous vacuum-assisted biopsy can achieve high diagnostic accuracy. For instance, in MRI-guided VAB series, success rates of 95–99% in targeting and sampling lesions have been reported. Likewise, stereotactic vacuum biopsies with 11–8G needles have demonstrated lower false-negative rates than older 14G core methods (Nakano et al., 2018). To ensure reliable diagnoses, radiologists correlate the pathology results with imaging, if there is any discordance (e.g. benign biopsy but imaging looks malignant), standard practice is to proceed to surgical excision.
-
High-risk or borderline lesions (B3 lesions): such as atypical ductal hyperplasia (ADH), lobular neoplasia, radial scars, or papillomas. These are often first discovered on core biopsy. Vacuum-assisted excision can then be performed to remove the lesion completely for more thorough examination, as an alternative to surgical biopsy. European guidelines endorse VAE in such scenarios to avoid overtreatment while still obtaining clear histology (Zouzos et al., 2025). The EnCompass system’s high-volume 7G sampling is well suited for this approach, as it can excise larger tissue chunks. A recent prospective randomized trial in Sweden (Karolinska Institutet) evaluated vacuum excisions of B2/B3 lesions <30 mm using 7G vs 10G needles. At 6-month follow-up, no residual lesion was seen in ~90% of cases excised with 7G and ~82% with 10G (a non-significant difference) (Zouzos et al., 2025). This suggests that even with the slightly smaller 10G probe, the majority of small lesions were completely removed. The study also highlighted that lesions presenting as microcalcifications (biopsied under stereotactic guidance) were more challenging to fully excise – about 30% of such cases had residual calcifications on follow-up imaging, versus only 2.5% residual rate for ultrasound-visible lesions. These findings reinforce that while VAE can often eliminate a target lesion, close imaging surveillance remains essential. EnCor EnCompass comes with the standard warning that tissue removal extent on imaging doesn’t always guarantee the histological margin is clear of disease. If a malignancy is diagnosed in an excised specimen, surgical follow-up is indicated per standard of care.
-
Benign tumor removal (e.g. fibroadenomas): Many patients with benign breast tumors seek non-surgical options to remove lumps that are enlarging or symptomatic. Vacuum-assisted removal through a small (~3 mm) skin incision can achieve this without general anesthesia or scarring. As noted, success rates for complete fibroadenoma excision approach 99% in experienced hands. The EnCompass system explicitly allows “complete removal of the imaged abnormality” when appropriate. However, it is approved for diagnostic use, not as a definitive cancer treatment – if cancer is found, the patient will still require standard surgical and oncologic management. Importantly, not all patients or lesions are candidates for percutaneous removal. The device is contraindicated when, in the physician’s judgment, the procedure would pose undue risk (for example, lesions too close to the chest wall or skin, or patients with bleeding disorders). Potential complications of any vacuum biopsy include bleeding (hematoma), infection, pain, or, very rarely, pneumothorax if the chest wall is inadvertently punctured. In clinical practice these events are uncommon and usually minor – in one 170-patient study of vacuum biopsies, only 1.2% had a small hematoma and none had serious complications (Bohan et al., 2021). Nonetheless, careful patient selection and technique are paramount to maintain safety.
Ultimately, the EnCor EnCompass clearance exemplifies how industry is responding to clinicians’ needs for precision and versatility in breast cancer diagnosis. The system’s launch comes amid a broader push in breast oncology toward earlier detection and less invasive management. By enabling efficient, accurate biopsies, and even complete removal of certain lesions, the technology may help patients avoid unnecessary surgery and get answers faster. Going forward, physicians will be watching closely how the EnCompass performs in real-world use.
Objective data on its impact (such as procedure time savings, patient comfort, or reduction in repeat biopsies) will be key to validating its benefits. For now, the device’s 510(k) clearance was supported by its substantial equivalence to existing vacuum biopsy platforms, along with bench and usability testing. As it enters the market in early 2026, BD’s EnCor EnCompass system stands poised to become a new all-in-one solution in breast biopsy, one that reflects the field’s continual drive toward earlier, easier, and more precise diagnosis of breast cancer.
Written by: Semiramida Nina Markosyan, Editor, OncoDaily Canada


