At OncoDaily Immuno-Oncology, we spotlight the week’s most influential conversations in cancer immunotherapy — from trial readouts and novel combinations to expert commentaries shaping the future of IO. This curated roundup distills insights from leading oncologists, researchers, and biotech innovators, offering a clear pulse on the science, strategy, and breakthroughs driving the field forward.
This Week’s Expert Highlights in Immuno-Oncology
Patrick Hwu (President and CEO at Moffitt Cancer Center):
” Why do some cancers resist anti-PD-1 immunotherapy?
- A new study in Nature Magazine shows that cancer-induced nerve injury (CINI) drives resistance. Tumors that damage nearby nerves trigger chronic inflammation, creating an immune-suppressive environment that blunts checkpoint blockade.
- Importantly, targeting nerve injury pathways — such as blocking IL-6 signaling — helped restore response in models, pointing to new strategies to overcome resistance.
Congratulations to co-corresponding author Kenneth Tsai of Moffitt Cancer Center, collaborators at MD Anderson Cancer Center and others for advancing this critical field.”
Jennifer S. Buell (President and Chief Executive Officer at MiNK Therapeutics):
“France Grants Reimbursed Compassionate Access (AAC) for Agenus’ BOT/BAL in Refractory MSS Metastatic Colorectal Cancer.
As a board member of Agenus, I am proud that our investigational combination of botensilimab plus balstilimab (BOT/BAL) is now available to eligible patients with refractory microsatellite-stable colorectal cancer under France’s compassionate access (Accès compassionnel, or AAC) framework.
This is more than a milestone for Agenus—it is a lifeline for patients and families facing one of the hardest-to-treat cancers. Published clinical data with BOT/BAL show durable responses and long term survival in this population, in fact, more than 42% of patients are alive beyond a median of 2 year follow-up. Through AAC, patients in France can now access therapy with 100% reimbursement, while hospitals and physicians receive clear guidance and support.
By contrast, in the U.S., compassionate use depends on single-patient INDs (SPINDs). These require FDA approval, institutional review board sign-off, and sponsor support on a case-by-case basis. The barrier is even higher because—unlike France—U.S. law prohibits reimbursement for investigational products prior to commercial approval. Medicare, Medicaid, and private insurers cannot pay for a therapy that has not received FDA approval, even if no alternatives exist. The intent is to protect patients and payers from unproven interventions, but in practice it means the cost falls to hospitals and/or companies. Many institutions, facing regulatory and financial strain, are now declining to support SPINDs altogether.
The result? U.S. patients often wait longer, face more obstacles, and sometimes miss out entirely on access to promising investigational therapies—while patients in France and elsewhere can receive care under nationally funded programs.
France’s AAC shows what’s possible when policy meets innovation. The U.S. (U.S. Department of Health and Human Services (HHS and FDA) must seek a better balance—one that ensures safety and evidence generation without closing the door on hope for patients with no other options.”
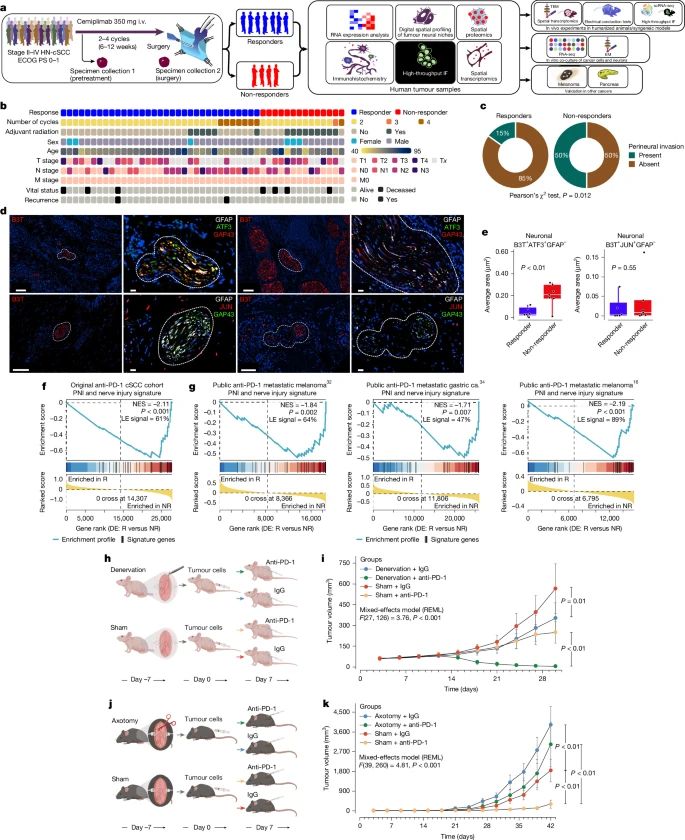
” Highlights from the 2nd Neuro-Immuno-Oncology Symposium by Institut Servier
Yesterday, I had the pleasure of co-chairing — alongside Guido KROEMER — the second symposium organized by Institut Servier, dedicated to Neuro-Immuno-Oncology, a rapidly evolving field where neuroscience, immunology, and oncology converge to explore new therapeutic avenues for central nervous system (CNS) tumors.
The first session brought together leading experts to discuss:
- The neuroanatomy of tumors
- Immune system interactions with neuroregeneration after injury
- CNS-tumor communications
- Circadian rhythms in brain tumors
- Neuro-controlled pathways that surprisingly influence tumor growth and survival
A standout moment was the keynote lecture by Dr. Moran Amit (MD Anderson Cancer Center), who shared groundbreaking research on the innervation of extracranial cancers — opening up a promising new field that could diversify treatment options for early-stage cancers.
The second session focused on immune-based therapies for brain tumors, including CAR-T cells, therapeutic vaccines, and oncolytic viruses. These innovative approaches continue to show encouraging results, especially as researchers refine strategies to deliver them safely and enhance their efficacy through combination therapies.
The symposium concluded with a strong consensus: cross-disciplinary collaboration is essential. All contributors expressed their commitment to keeping this topic high on their agenda and to building more structured interactions to accelerate progress.
A heartfelt thank you to all participants for their insights, energy, and dedication!”
Iannis Aifantis (Chairman of the Department of Pathology at NYU School of Medicine):
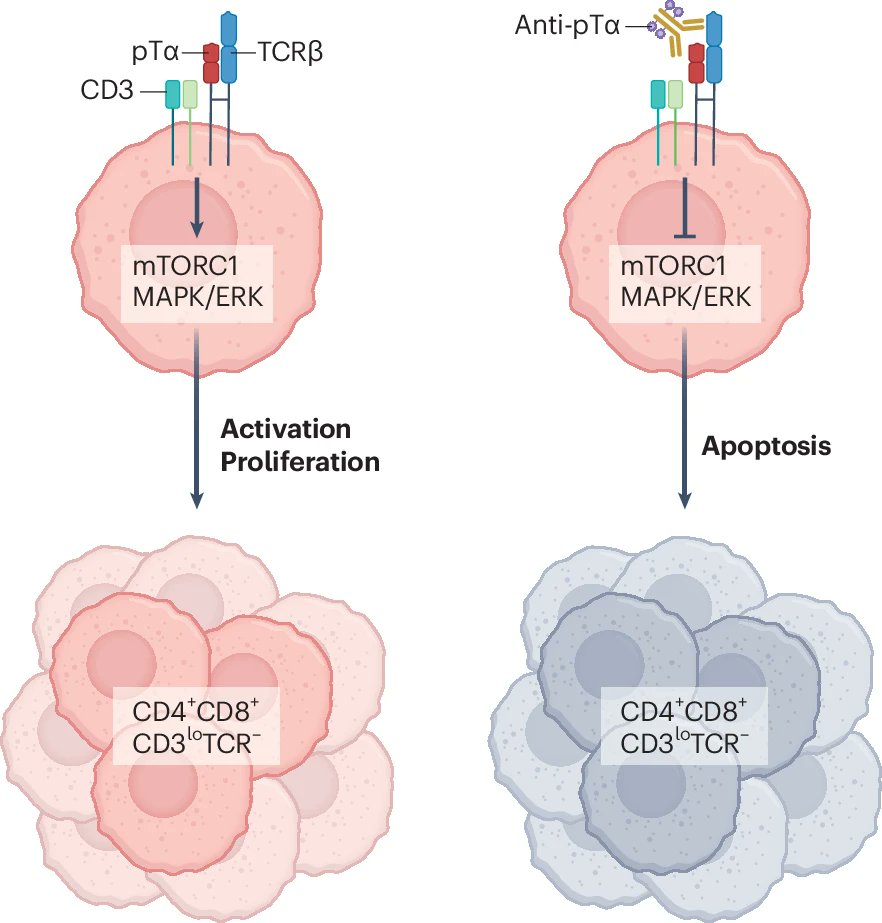
“You can never forget your first love!!
It took me back almost 25 years but it was a lot of fun to write (together with Ali Rosen in the lab) this News and Views for the fantastic story by Maria Toribio and colleagues on the pre-TCR as an immunotherapy target in T cell leukemia.”
Minhua Chu (Managing Partner at TransitionValue Partner):
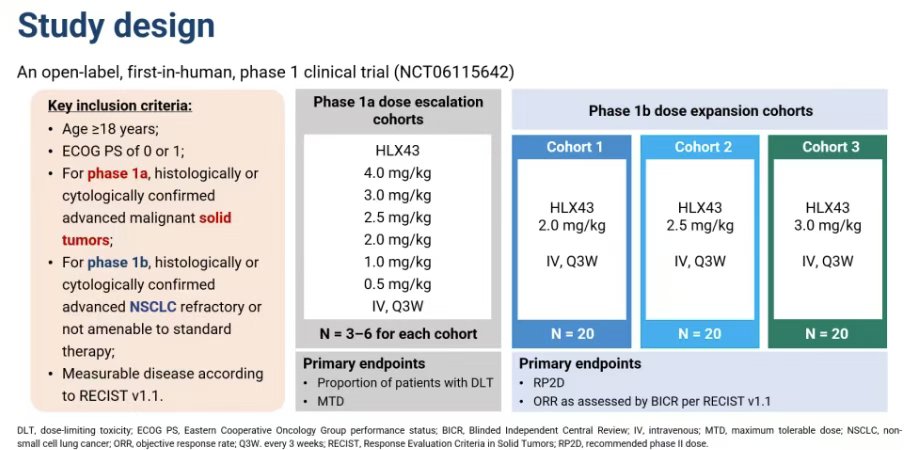
” Henlius reported the Phase 1 clinical data for its PD-L1 ADC HLX43 at WCLC25. In Phase Ia, patients received HLX43 at doses of 0.5 mg/kg, 1 mg/kg, 2 mg/kg, 2.5 mg/kg, 3 mg/kg, or 4 mg/kg once every three weeks.
In Phase Ib, patients with advanced/metastatic NSCLC refractory to standard treatments received intravenous injections of HLX43 at doses of 2 mg/kg, 2.5 mg/kg, or 3 mg/kg once every three weeks. ”
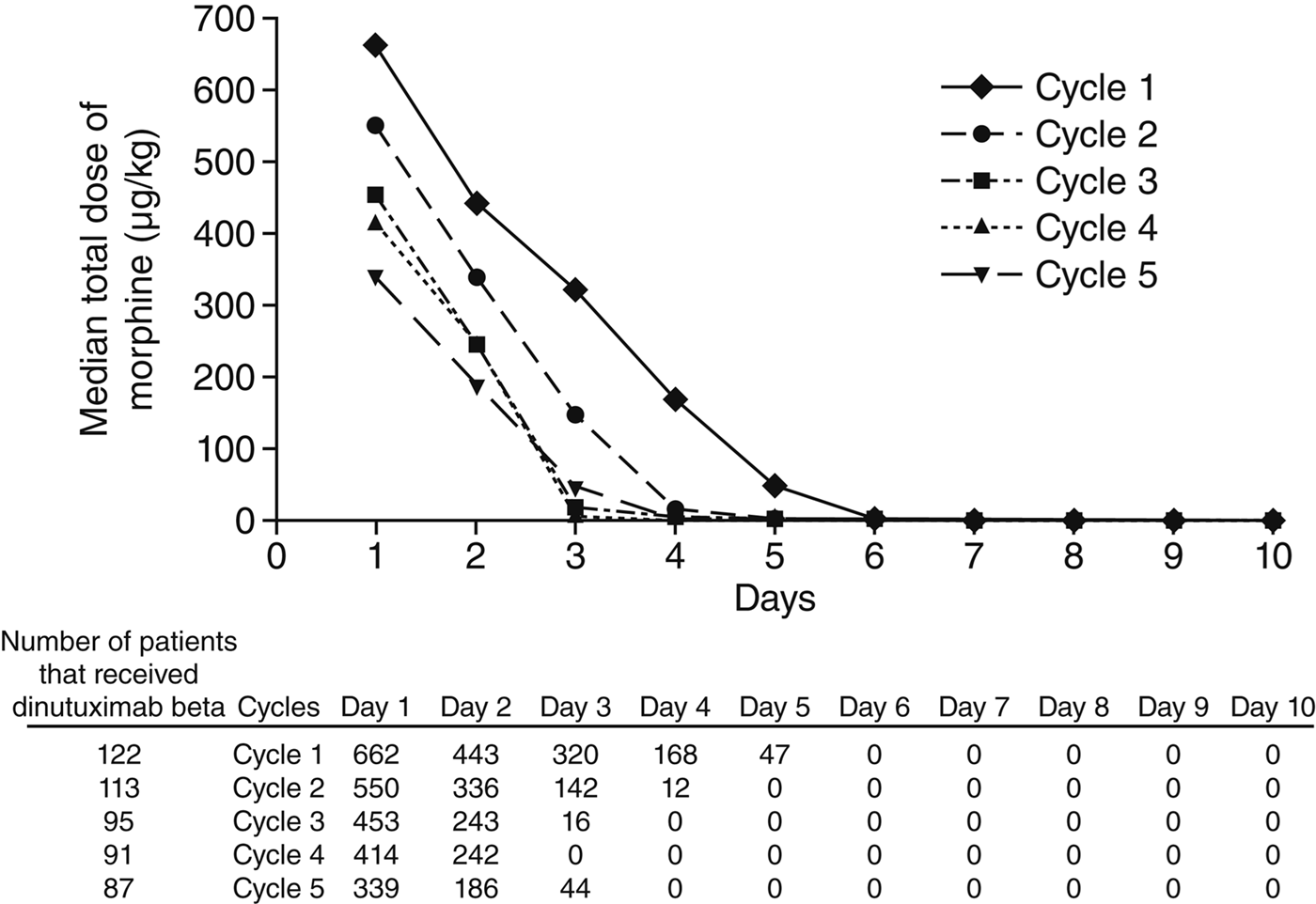
“Phase 1/2 Study: FCGR polymorphism correlates with dinutuximab beta outcomes.”
Masahiro Torasawa (Medical Oncologist at Juntendo University):
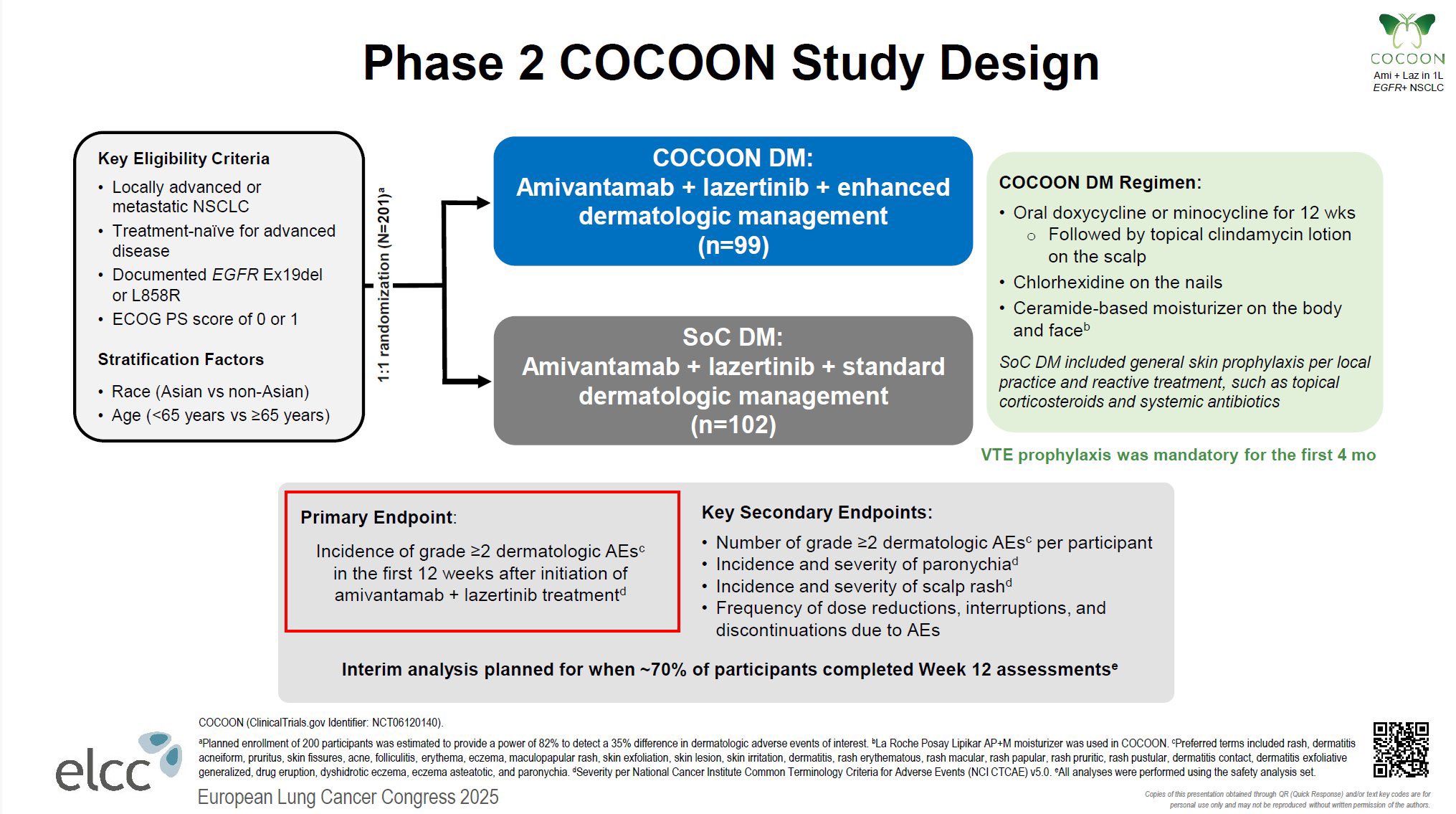
“How to Reduce Skin Toxicity of Amivantamab + Lazertinib? – COCOON Trial (Ph2)
Key Results
- Significantly reduced Gr ≥2 dermatologic AEs within 12 weeks (42% vs 75%, p<0.0001).
- Less impact of skin symptoms on QoL.
- Delayed time to onset of severe skin AEs (median 1.0 mo → 3.5 mo).
- Limited effect on paronychia (21% vs 23%; p=0.76)
Discussion
- A simple, widely available prophylactic regimen greatly improves tolerability in clinical practice.
- Essential supportive care to maximize the efficacy benefits seen in the MARIPOSA trial.
- New strategies are needed to manage paronychia.
- Prophylactic anticoagulation for the first 4 months is also effective for VTE risk.
Italiano Antoine (Head of Precision Medicine at Gustave Roussy):
” CD40 Targeting and TLS Formation – A New Horizon in Cancer Immunotherapy
One of the most exciting frontiers in cancer immunotherapy today is the ability to actively induce tertiary lymphoid structures (TLS) within tumors. TLS are organized immune hubs where B cells, T cells, and dendritic cells meet, interact, and generate a local antitumor immune response. Their presence has consistently correlated with better outcomes across cancers – but the big challenge has been: how do we therapeutically trigger them?
- A new Cancer Cell paper by Osorio et al. provides a breakthrough:
An Fc-optimized CD40 agonist (2141-V11), given intratumorally, was able to induce TLS formation and drive systemic antitumor immunity – with complete responses in patients with melanoma and breast cancer. This is one of the first clinical demonstrations that CD40 targeting can actively build TLS inside human tumors.
But this is not an isolated example. For instance, Melero et al. (ESMO 2024, Annals of Oncology) reported that targeted delivery approaches such as FAP-CD40 agonists (RO7300490) can restrict CD40 stimulation to the tumor stroma. In their study, this strategy not only promoted dendritic cell maturation and local immune activation but also led to TLS induction within tumors—further supporting the idea that CD40 agonism can reprogram the tumor microenvironment into an immune-permissive niche.
Why this matters:
TLS are not just biomarkers – they can be a therapeutic goal.
- CD40 agonism provides a direct way to reprogram the tumor microenvironment into an immune-permissive niche.
- By safely inducing TLS, we can potentially unlock durable, systemic antitumor immunity even from local interventions.
This evolving field shows how CD40 agonists, when properly engineered and delivered, can transform “cold” tumors into self-sustaining immune ecosystems. TLS-inducing strategies may become a cornerstone for next-generation immunotherapies.
Exciting times ahead – with next-phase studies emerging across multiple tumor types.”
Enrique Grande (Head of Oncology at MD Anderson Cancer Center):
” Consolidative surgery in advanced UC
New data show that after Enfortumab Vedotin ± ICI, surgery may offer:
- 82% downstaging
- 43% ypT0N0
- 79% discontinued systemic therapy
- 89% DFS in the curative-intent group”

Kamal S. Saini (Executive Medical Director and Head of European Medical Oncology Team at Fortrea Inc.):
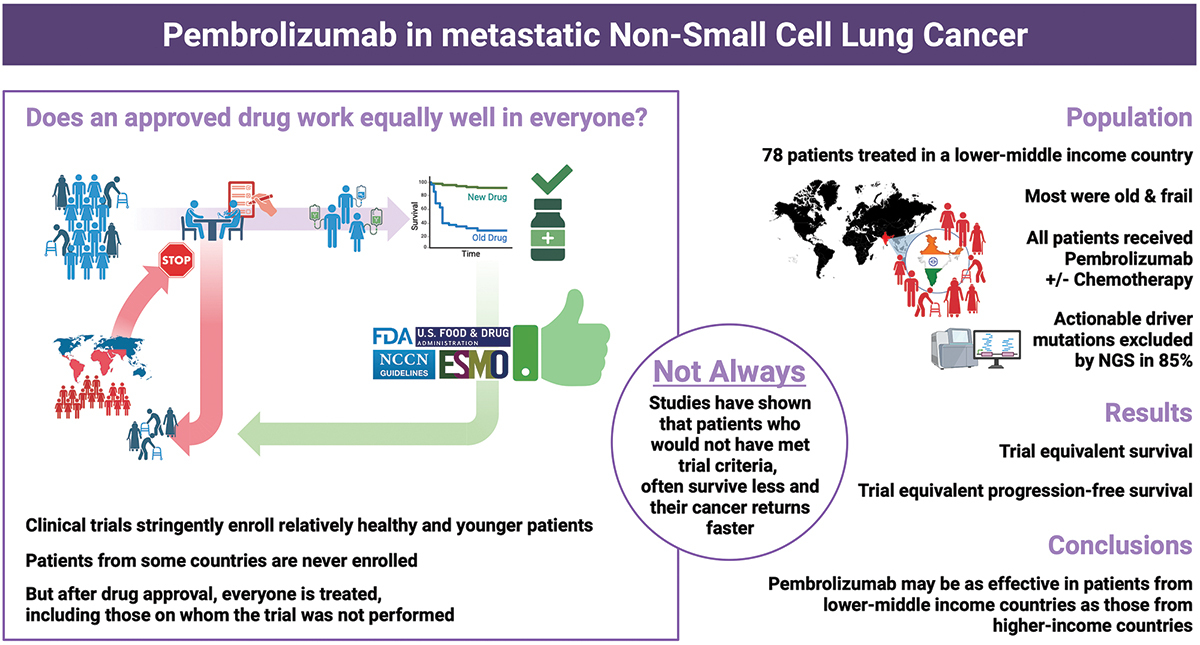
” NSCLC real world data from India, in collaboration with Dr Ullas Batra and his distinguished team: “First-line pembrolizumab for metastatic NSCLC in lower-middle-income countries: bridging the efficacy-effectiveness gap”. Out now in Immunotherapy (Taylor and Francis Group).
78 patients with advanced NSCLC received pembrolizumab-based first-line therapy; and after a median follow-up of 27 months, median OS was 21 months and median PFS 6.3 months. Real-world hashtag LMIC data demonstrated comparable effectiveness of pembrolizumab-based therapy in mNSCLC despite a higher proportion of adverse prognostic factors.”
Written by Toma Oganezova, MD, Editor-in-Chief of OncoDaily IO


