
How Could Robert F. Kennedy Jr.’s $500M mRNA Vaccine Funding Cut Impact Cancer Research?
Robert F. Kennedy Jr.’s decision to halt approximately $500 million in funding for 22 messenger RNA (mRNA) vaccine projects under the Biomedical Advanced Research and Development Authority (BARDA) marks a pivotal shift in U.S. biotechnology strategy. While the move primarily affects infectious disease programs, oncology researchers warn that it could indirectly disrupt the rapidly advancing field of mRNA cancer vaccines, which have shown promising results in early and mid-stage clinical trials for melanoma, pancreatic cancer, and other malignancies.
Robert F. Kennedy Jr. justifies the change by claiming that mRNA vaccines “fail to protect effectively against upper respiratory infections like COVID and flu” and can “encourage new mutations and actually prolong pandemics”. He states that HHS will now redirect funding towards “safer, broader vaccine platforms” like whole-virus vaccines, which he believes offer more enduring protection. While some final-stage mRNA contracts will be allowed to conclude to preserve prior taxpayer investment, no new mRNA projects will be initiated.
However, Robert F. Kennedy Jr.’s scientific claims are largely disputed by the broader medical and public health community. Experts, including former FDA officials and leading vaccinologists, emphasize that mRNA vaccines were instrumental in slowing the COVID-19 pandemic and saving millions of lives due to their remarkable safety profile and rapid development cycle.
They point out that viruses naturally mutate, regardless of vaccination, and warn that defunding mRNA research poses a “self-inflicted vulnerability” that will severely hamper the U.S.’s ability to respond swiftly to future pandemics. The same manufacturing speed, adaptability, and safety profile that allowed for pandemic-scale deployment are essential in mRNA cancer vaccines, particularly those encoding patient-specific neoantigens for use alongside checkpoint inhibitors. Removing infrastructure investment in infectious-disease mRNA programs risks collateral delays in oncology trials that depend on identical lipid nanoparticle, plasmid DNA, and quality-control pipelines.
This decision is the latest in a series of controversial actions taken by Robert F. Kennedy Jr., a long-standing vaccine critic. His tenure has also seen the firing and replacement of the expert committee that issues official government immunization recommendations, and the removal of the COVID-19 vaccine from the CDC’s recommended immunization schedule for healthy children and pregnant women. Critics argue these changes will erode public trust in vaccines and undermine national health security.
What is mRNA Vaccine?
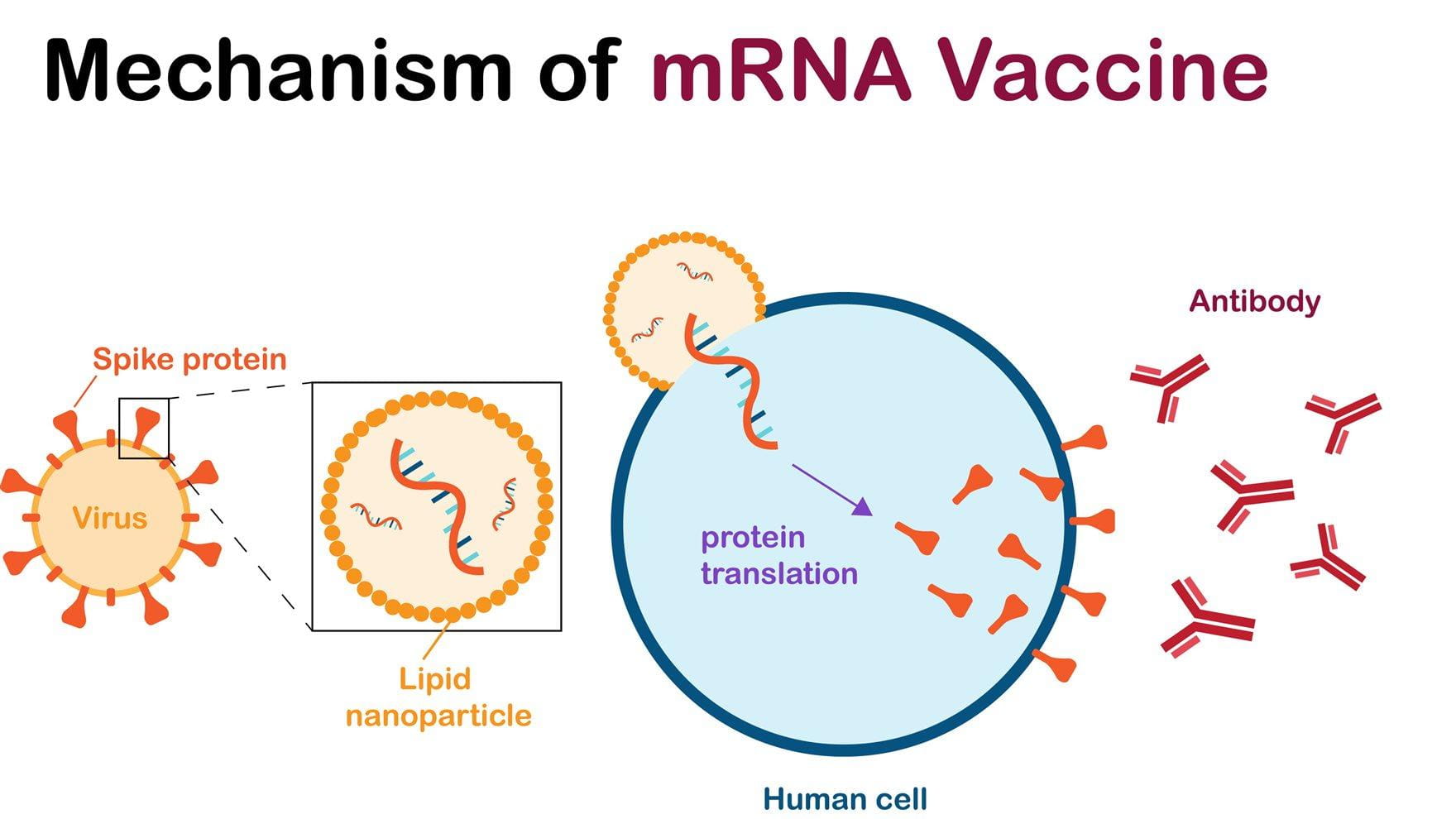
mRNA vaccines are a relatively new type of vaccine that work by giving your body’s cells genetic instructions (messenger RNA, or mRNA) to produce a harmless piece of a virus, typically a protein found on its surface. Your immune system recognizes this protein as foreign and then learns to produce antibodies and immune cells that can quickly fight off a real infection if you encounter the virus later.
Unlike traditional vaccines that use weakened or inactivated viruses, mRNA vaccines do not contain any live virus and cannot cause the disease they are designed to protect against. The mRNA itself is temporary and breaks down in the body after delivering its instructions, without altering your DNA. In cancer, mRNA vaccines are not preventive but therapeutic—delivering sequences for tumor-specific or tumor-associated antigens to elicit robust cytotoxic T-lymphocyte responses. This mechanism complements PD-1/PD-L1 blockade, with the aim of eradicating micrometastatic disease or enhancing responses in advanced tumors.
How Will This Affect The US Healthcare?
mRNA technology is known for its speed in vaccine development, which was critical during the COVID-19 pandemic. By shifting away from this platform, the U.S. may significantly delay its ability to rapidly produce new vaccines in response to future emerging pathogens or viral variants. Experts warn this could leave the country without sufficient vaccine supplies for months longer than other nations, potentially leading to increased illness and mortality during the next public health crisis.
This move could cede U.S. leadership in a crucial biotechnological field to other countries that continue to invest heavily in mRNA research, potentially making the U.S. dependent on foreign innovation for breakthrough treatments.
Impact on Vaccine Innovation
The cancellation of existing contracts and the halt of new funding will directly curtail ongoing research into mRNA vaccines for various diseases, including flu, bird flu, and potentially even other infectious diseases. Chilling Effect on Broader mRNA Research: While the announcement focuses on prophylactic vaccines for infectious diseases, some experts worry it could have a “chilling effect” on the broader development of mRNA technology for other medical applications, such as cancer immunotherapies and treatments for genetic diseases, where it shows immense promise. This could discourage private investment and deter researchers from pursuing mRNA-based solutions.
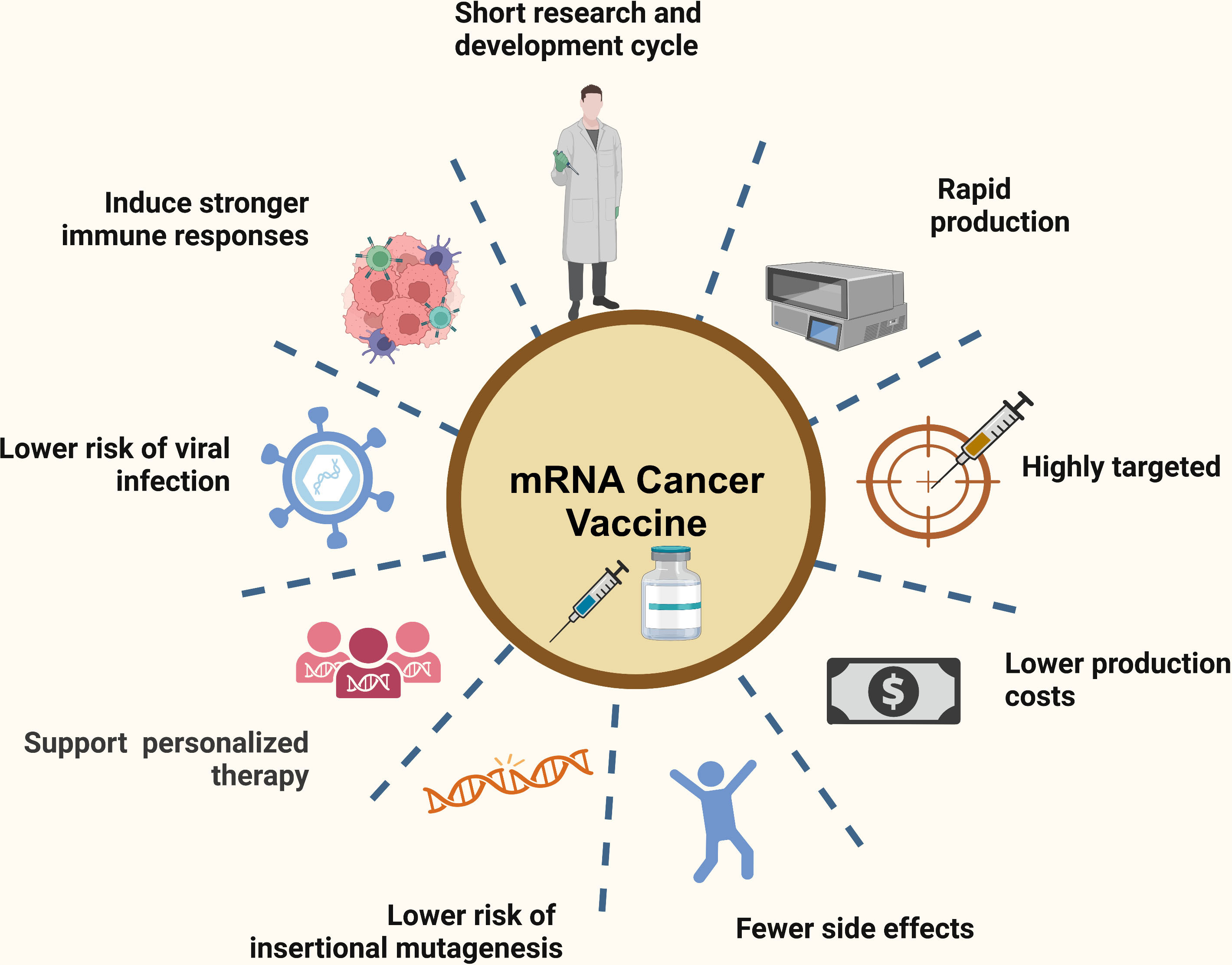
Source: Wang et al; Recent advances in mRNA cancer vaccines: meeting challenges and embracing opportunities; Published in Frontiers, 2023
Shift to “Older” Technologies: The stated shift towards “safer, broader vaccine platforms” like whole-virus vaccines, while effective for certain applications, may represent a step back from a technology that offers greater adaptability and speed for rapidly evolving threats. For example, mRNA-4157 (V940) in melanoma and autogene cevumeran in pancreatic cancer both require rapid synthesis and formulation of personalized RNA payloads. Any bottleneck in plasmid production or lipid nanoparticle encapsulation capacity could extend treatment initiation timelines by several weeks, potentially diminishing therapeutic benefit in high-risk patients.
Impact on Cancer Vaccine Development
mRNA platforms have become central to next-generation oncology therapeutics, enabling rapid, patient-specific neoantigen design and robust cytotoxic T-cell activation. In the randomized phase 2b KEYNOTE-942 trial (Weber et al, Lancet, 2024), the individualized mRNA-4157 (V940) vaccine combined with pembrolizumab in resected high-risk melanoma reduced the risk of recurrence or death by 44% compared with pembrolizumab alone (HR 0.56; 95% CI 0.31–0.99) at a median 23-month follow-up, leading to multiple ongoing phase 3 programs. In a phase 1 study of autogene cevumeran (BNT122) in resected pancreatic ductal adenocarcinoma (Lopez et al, Nature, 2023), patients who mounted vaccine-induced CD8⁺ T-cell responses achieved markedly prolonged recurrence-free survival (median not reached vs 13.4 months for non-responders) at approximately three years.
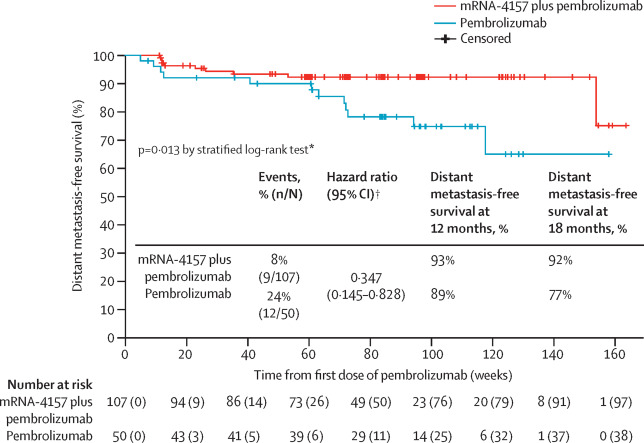
Kaplan-Meier estimates for distant metastasis-free survival in the intention-to-treat population
Source: Weber, Jeffrey S et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study; The Lancet, Volume 403, Issue 10427, 632 – 644
BioNTech’s BNT111, a fixed-sequence multi-antigen mRNA vaccine for advanced melanoma, has shown durable antigen-specific T-cell responses and early disease-control signals in phase 1/2 trials. Curtailing U.S. federal support for mRNA infrastructure risks extending individualized vaccine manufacturing timelines by several weeks, raising production costs, and shifting late-stage oncology development to regions with stronger mRNA capacity such as the EU and China.
Clinical Pipeline at Risk
As of late 2024, at least 36 mRNA oncology therapeutics were in active development, with more than 120 clinical trials registered globally. These programs depend on rapid, high-quality production of lipid nanoparticle formulations and individualized neoantigen payloads—processes supported by public–private partnerships and advanced manufacturing networks in the United States. BARDA’s withdrawal from infectious-disease mRNA contracts could indirectly erode these capabilities, increasing trial costs and limiting patient access to experimental cancer vaccines. Loss of BARDA support could increase per-patient manufacturing costs for individualized mRNA vaccines by 20–40% and extend production timelines by 2–6 weeks, based on current GMP capacity constraints reported in industry surveys.
mRNA-4157/V940 was included in OncoDaily 2024 Special Edition of the 10 Most Promising Cancer Drugs
Erosion of Public Trust and Increased Vaccine Hesitancy
Undermining Scientific Consensus: The Health Secretary’s public claims questioning the safety and efficacy of mRNA vaccines, which contradict the vast body of scientific evidence and the consensus of the medical community, are likely to further fuel vaccine skepticism and misinformation.
Decreased Vaccination Rates: This could lead to a decline in vaccination rates for not only mRNA-based vaccines but potentially other recommended immunizations as well, increasing the risk of outbreaks of preventable diseases like measles, which have already seen a resurgence in some areas.
Politicization of Public Health: The highly politicized nature of this decision, particularly given the Secretary’s history as a vaccine critic, risks further politicizing public health initiatives and recommendations, making it harder for the public to trust official health guidance. Public skepticism toward mRNA respiratory vaccines can generalize to oncology settings, potentially lowering accrual in cancer vaccine trials. This is particularly problematic in rare tumor types where recruitment speed is already a limiting factor for statistical power.
Scientific and Public Health Implications
Unlike prophylactic vaccines against respiratory viruses, therapeutic mRNA cancer vaccines are designed to generate targeted immune responses against tumor-specific or tumor-associated antigens. This approach complements checkpoint inhibitors and chemotherapy, offering a mechanism to personalize cancer treatment. Reductions in government investment may hinder innovation in a field that requires rapid adaptation of mRNA payloads to evolving tumor mutational landscapes, ultimately affecting survival outcomes in malignancies with historically poor prognoses. Therapeutic mRNA vaccines can be rapidly re-engineered to incorporate novel neoantigens arising from tumor evolution or treatment resistance. Reduced investment may slow the adaptive cycle needed to maintain efficacy in cancers with high mutational burden.
Counterarguments/Nuances
It’s noted that “other uses of mRNA technology” within HHS are not currently impacted. This suggests that research into mRNA for non-vaccine applications (like cancer treatments) may continue, though the broader impact on the field remains a concern for some, as the shared manufacturing, regulatory, and talent infrastructure means oncology research will likely experience indirect consequences. In 2024–2025, randomized phase 2 data in resected melanoma showed that individualized mRNA-4157 (V940) plus pembrolizumab significantly improved recurrence-free survival, with phase 3 trials now in progress. BioNTech’s BNT111 in advanced melanoma and autogene cevumeran in pancreatic cancer have each produced durable immune responses and survival signals, supporting ongoing randomized testing. With at least 36 mRNA oncology therapeutics in development and over 120 trials registered worldwide, reduced federal support risks delaying pivotal timelines, increasing production costs, and shifting late-stage development to countries with stronger mRNA infrastructure
The long-term impact of halting $500 million in BARDA-funded mRNA projects will depend on how quickly alternative funding and manufacturing capacity can be secured. While non-vaccine mRNA research within HHS may continue, the shared infrastructure between infectious-disease and oncology applications makes some degree of disruption likely. With more than 120 active cancer vaccine trials worldwide—including late-phase studies in melanoma and pancreatic cancer—the trajectory of this technology will hinge on whether U.S. programs can maintain their pace of innovation amid shifting federal priorities. The coming years will reveal whether domestic research networks can adapt to these changes or whether leadership in mRNA-based cancer therapeutics will migrate to countries sustaining or expanding their investments.
Written by Aren Karapetyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023

