Original Study Title:MARIPOSA: A Phase 3 International Randomized Trial of Amivantamab–Lazertinib versus Osimertinib in Previously Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer
Authors: James Chih-Hsin Yang, M.D., Ph.D.; Shun Lu, M.D., Ph.D.; Hidetoshi Hayashi, M.D., Ph.D.; Enriqueta Felip, M.D., Ph.D.; Alexander I. Spira, M.D., Ph.D.; Nicolas Girard, M.D., Ph.D.; Yu Jung Kim, M.D., Ph.D.; Se-Hoon Lee, M.D., Ph.D.; Yurii Ostapenko, M.D., Ph.D.; Pongwut Danchaivijitr, M.D.; Baogang Liu, M.D.; Adlinda Alip, M.D.; Ernesto Korbenfeld, M.D.; Josiane Mourão Dias, M.D.; Benjamin Besse, M.D., Ph.D.; Antonio Passaro, M.D., Ph.D.; Ki-Hyeong Lee, M.D.; Hailin Xiong, M.D.; Soon-Hin How, M.D.; Ying Cheng, M.D.; Gee-Chen Chang, M.D., Ph.D.; Hiroshige Yoshioka, M.D., Ph.D.; Michael Thomas, M.D.; Danny Nguyen, M.D.; Sai-Hong Ignatius Ou, M.D., Ph.D.; Sanjay Mukhedkar, M.D.; Kumar Prabhash, M.D., D.M.; Manolo D’Arcangelo, M.D.; Jorge Alatorre-Alexander, M.D.; Juan Carlos Vázquez Limón, M.D.; Sara Alves, M.D.; Daniil Stroyakovskiy, M.D.; Marina Peregudova, M.D., Ph.D.; Mehmet Ali Nahit Şendur, M.D., Ph.D.; Ozan Yazici, M.D.; Raffaele Califano, M.D.; Vanesa Gutiérrez Calderón, M.D.; Filippo de Marinis, M.D.; Sang-We Kim, M.D., Ph.D.; Shirish M. Gadgeel, M.D., Ph.D.; Scott Owen, M.D.; John Xie, Ph.D.; Tao Sun, Ph.D.; Jaydeep Mehta, Ph.D.; Raja Venkatasubramanian, Ph.D.; Mariah Ennis, M.S.; Elizabeth Fennema, M.A.; Mahesh Daksh, Ph.D.; Amy Roshak, B.S.; Julie Man, M.S.; Roland E. Knoblauch, M.D., Ph.D.; Joshua M. Bauml, M.D.; Mahadi Baig, M.D.; Sujay Shah, M.D.; Seema Sethi, D.O.; and Byoung Chul Cho, M.D., Ph.D., for the MARIPOSA Investigators
First-Line Breakthrough for EGFR+ NSCLC
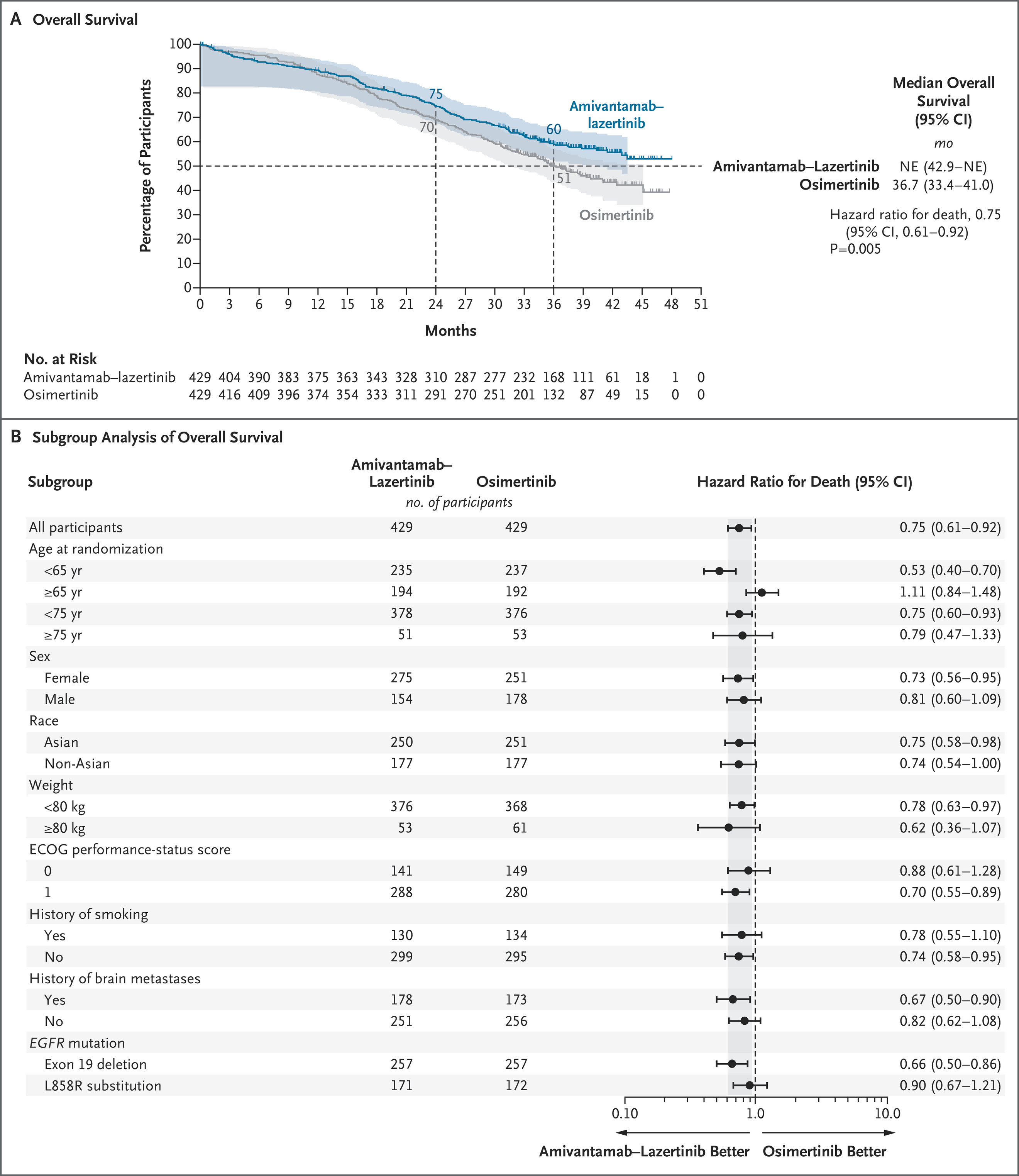
The protocol-specified final overall survival analysis from the MARIPOSA trial confirms that the bispecific antibody–TKI combination amivantamab–lazertinib offers a significant survival advantage over osimertinib in previously untreated EGFR exon 19 deletion or L858R advanced NSCLC.
At 3 years, overall survival was 60% with amivantamab–lazertinib vs. 51% with osimertinib, with a hazard ratio (HR) for death of 0.75 (95% CI, 0.61–0.92; P=0.005).
Study Design and Methodology
The phase 3 MARIPOSA trial enrolled 858 adults with previously untreated, EGFR-mutated (Ex19del or L858R) advanced or metastatic non–small-cell lung cancer. Participants were randomly assigned in a 2:2:1 ratio to receive either the combination of amivantamab plus lazertinib, standard-of-care osimertinib, or lazertinib monotherapy. Amivantamab was administered intravenously once weekly for the first four weeks and every two weeks thereafter, while both tyrosine kinase inhibitors—lazertinib and osimertinib—were taken orally once daily (osimertinib at 80 mg). The trial’s primary efficacy endpoint, previously reported, was progression-free survival; the protocol-specified final analysis focused on overall survival as a key secondary endpoint.
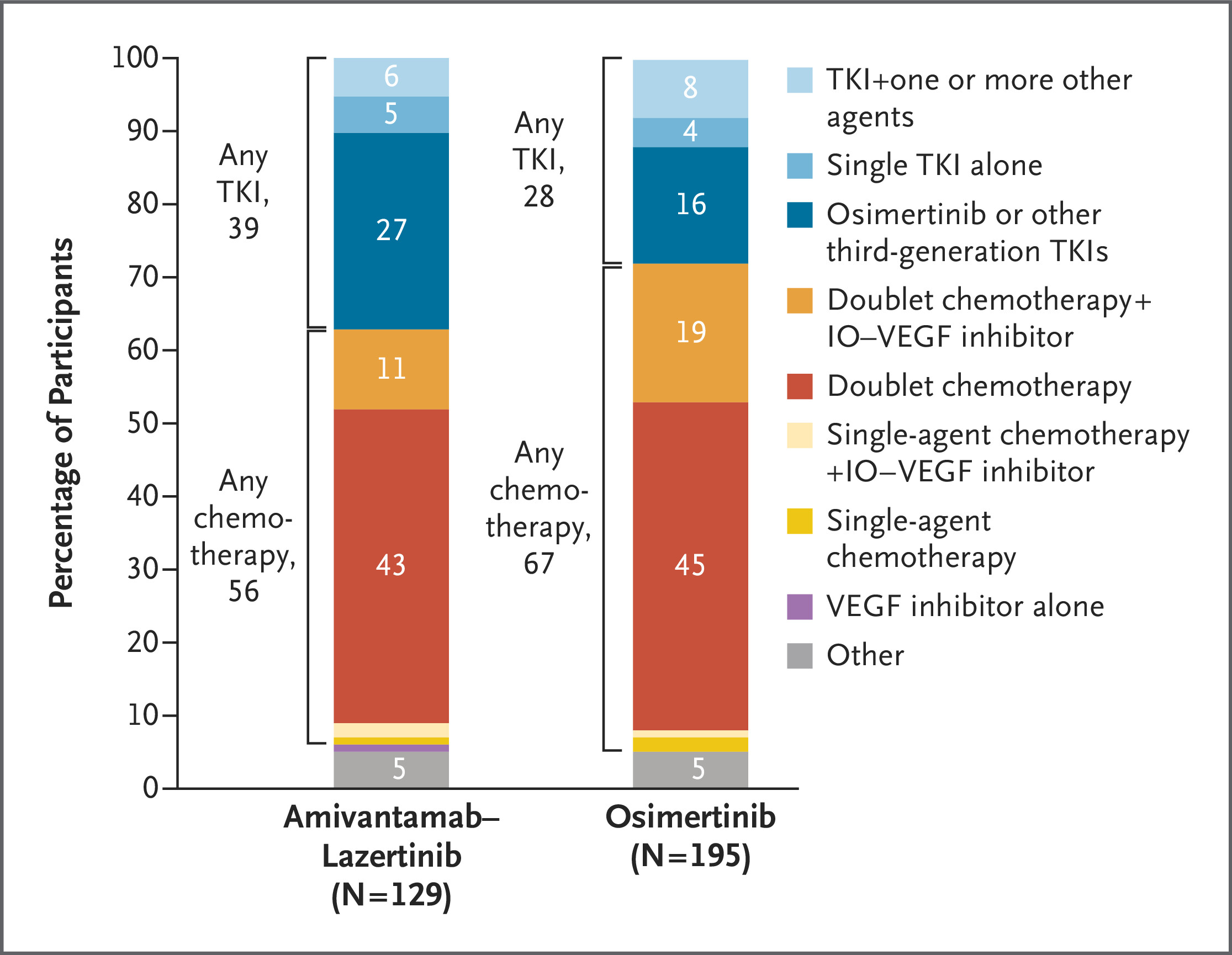
Key Findings
- Overall Survival: Median OS not reached (amivantamab–lazertinib) vs. 36.7 months (osimertinib)
- 3-Year OS: 60% vs. 51%
- Symptomatic Progression: 43.6 vs. 29.3 months (HR 0.69)
- Time to Subsequent Therapy: 30.3 vs. 24.0 months (HR 0.76)
- Intracranial Outcomes: Similar intracranial response (~70%), but longer durability with amivantamab–lazertinib
Safety Profile
- Grade ≥3 AEs: More frequent with amivantamab–lazertinib (80% vs. 52%)
- Common events: rash, paronychia, venous thromboembolism, infusion reactions
- No new safety signals emerged; supportive care and SC amivantamab may mitigate tolerability issues
Clinical Implications
Amivantamab–lazertinib is the first regimen to surpass osimertinib in overall survival for EGFR-mutated NSCLC. By co-targeting EGFR and MET, the combination delays resistance and extends survival, projecting >12 months OS benefit over osimertinib.


