The ESMO Congress 2024 is ongoing in Barcelona, from September 13 to 17, 2024, at the Fira Barcelona Gran Via.
This significant event, organized by the European Society for Medical Oncology (ESMO), attracted over 30,000 participants, including healthcare professionals, researchers, patient advocates, and industry representatives from around the globe.
The congress featured nearly 300 sessions, presenting over 2,000 abstracts, with a focus on the latest advancements in cancer research and treatment. Key topics included new therapeutic combinations for various cancers, the long-term effects of immunotherapy, and innovative approaches integrating artificial intelligence into cancer care.

Here are some highlights from ESMO Congress 2024 Day 1:
European Society for Medical Oncology:
“The Opening Ceremony marks the official start of ESMO24. From 13 to 17 September, delegates will hear from inspiring speakers, delve into new research, and connect with peers who share their same passion for finding improved ways to care for patients with cancer.
ESMO enables oncologists to enhance their knowledge and gain new insights, supporting a well-rounded approach to Patient Care.”

“First session of my ESMO24 is gonna be hard to top. Talking all things trial design, drug approval and reimbursement, and patient-centred outcomes. Packed room and engaging Q+A will tell you the common sense oncology movement is picking up steam!”

“Agree with Tom Powles, Uromigos very exciting compound that merits further investigation in advanced UC as monotherapy and combos! Great thoughtful discussion by Jonathan Rosenberg. I recall our work in my postdoc.”

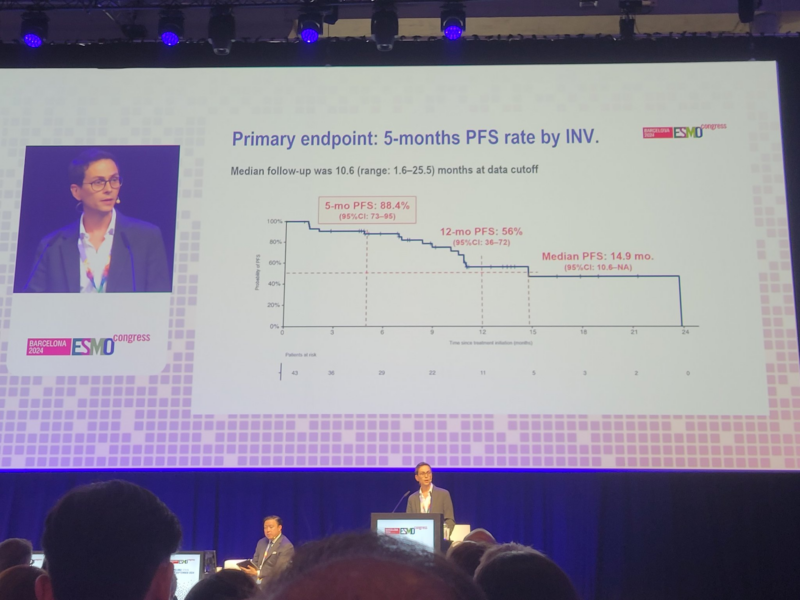
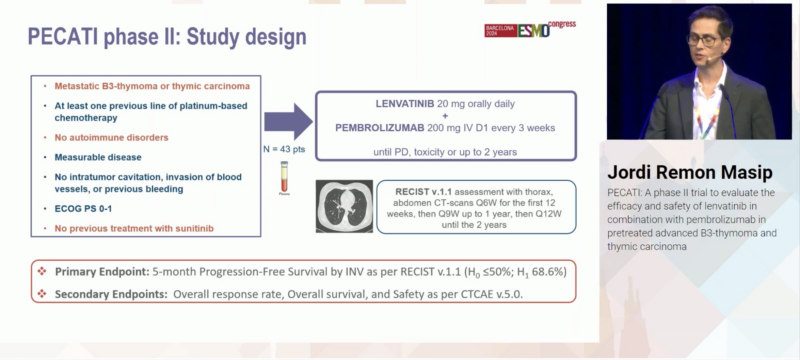
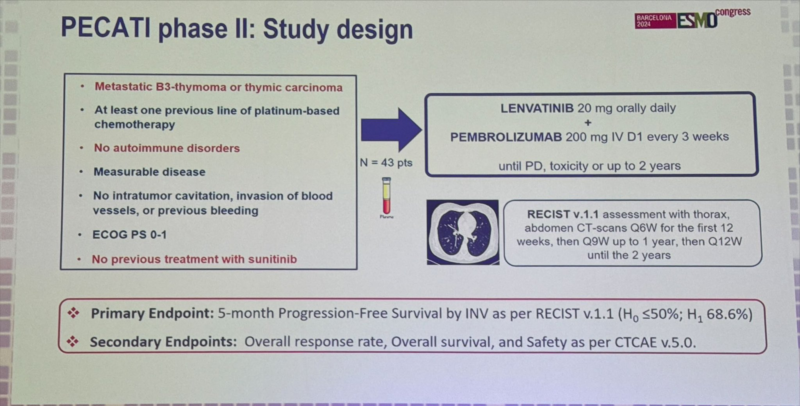
“Dr. Jordi Remon presents ph2 single-arm PECATI Trial of subsequent line pembro/lenvatinib in B3 thymoma and thymic carcinoma.
Study for PFS endpnt with promising 5mo PFS 88.3% & mPFS 14.9mo.
Great to see promising combinations in these rare tumors with great need.”
“ESMO 2024 kicks off! Let’s unite in advancing oncology, bringing together cutting-edge research and innovation to improve patient care.”

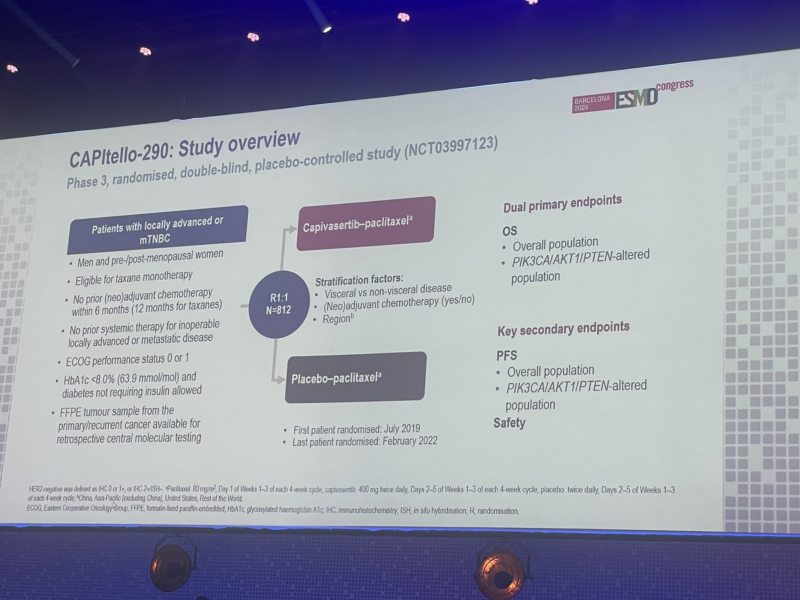
“Negative results for Capivasertib + paclitaxel as first-line treatment of metastatic TNBC in the CAPItello-290 phase III trial.
Primary endpoint (OS) not met.”
“What a pleasure and privilege to join with ESMO24 Meeting Chair Rebecca Dent and incoming President Fabrice Andre for YOC leadership discussion.
Successful careers and leadership comes in all shapes and sizes. Stay strong!”

“Olverembatinib, in ESMO, showed benefits for GIST patients with SDH deficiency, interesting results for an orphan disease.
A new opportunityCésar Serrano García?”

“Discusssnt Stephen Liu; ADRIATIC new global SOC; half offered PCI. Benefit to be evaluated in ongoing trials carbo seems better than cis but confounders.
Re Bay> large study? Or small biomarker study.”

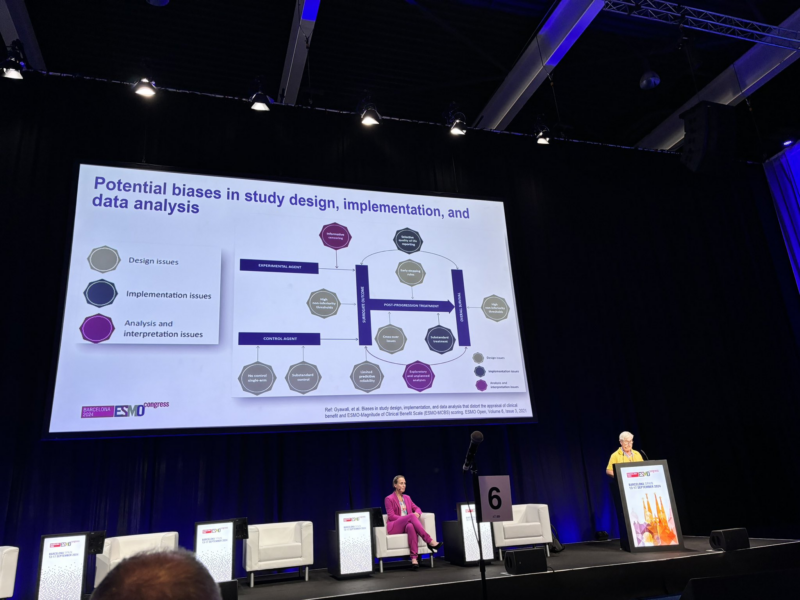
“First talk of the session: Nathan Cherny, the ‘father of MCBS’ highlights how biases are being addressed in the MCBS tool.”
“Selfie with the Common Sense Oncology organizers and ASCO folks Bishal Gyawali, Julie Gralow, Lynn Schuchter, at ESMO24. The local hosts kindly offered me a ride to the nearest metro.”

“Jordi Remon elegantly presenting PECATI phase 2 lenvatinib plus pembro in pretreated B3-thymoma and thymic carcinoma. Primary endpoint met 5-mo PFS 88%. Promising 12-mo OS 85%. Higher dose intensity associated with better outcomes. Manageable safety BUT irAE 14%.”
“The best way to start ESMO2024 for neuroendocrine community! Amazing session, brilliant speakers, vibrant discussions. Lovely friends and colleagues and more
- CMV-118 in progressive NET
- CABONEN in low G3 NEN
- NETTER-2 and new insights
- Carcinoid heart disease and NET.”

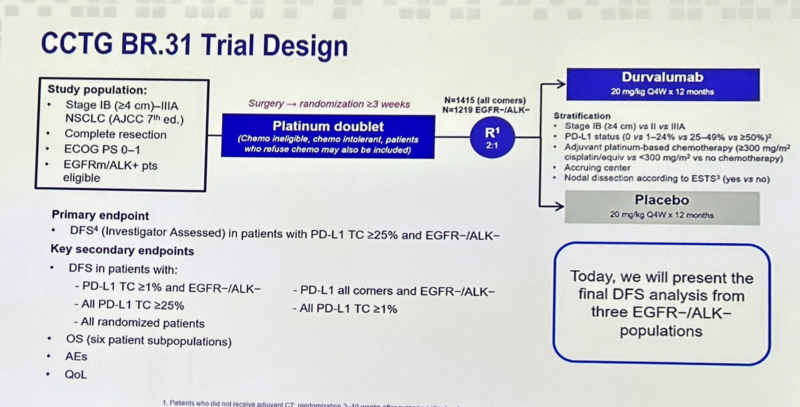
“BR31 by Dr. Glen Goss, a herculean academic effort examining adjuvant durvalumab for resected lung cancer sadly negative, pushes the narrative even more toward neoadj/periop, the results by PD-L1 suggest this was a study destined to be negative.”
“ESMO24 ThymicCa: Lenvatinib+Pembro meaningful ORR23% mPFS 14.9 mo in 2+ line of therapy.”
“Big thanks to Giuseppe Curigliano for the insightful mentorship session on the Career Path ESMO24.
Very familiar chair too, Deniz Can Guven.
It was an inspiring and valuable mentorship session.”

“Fantastic talk by brilliant Toni Choueiri on Ti-Nivo2 trial! Second trial after Contact03 with NO benefit with ICI rechallenge in mRCC!”
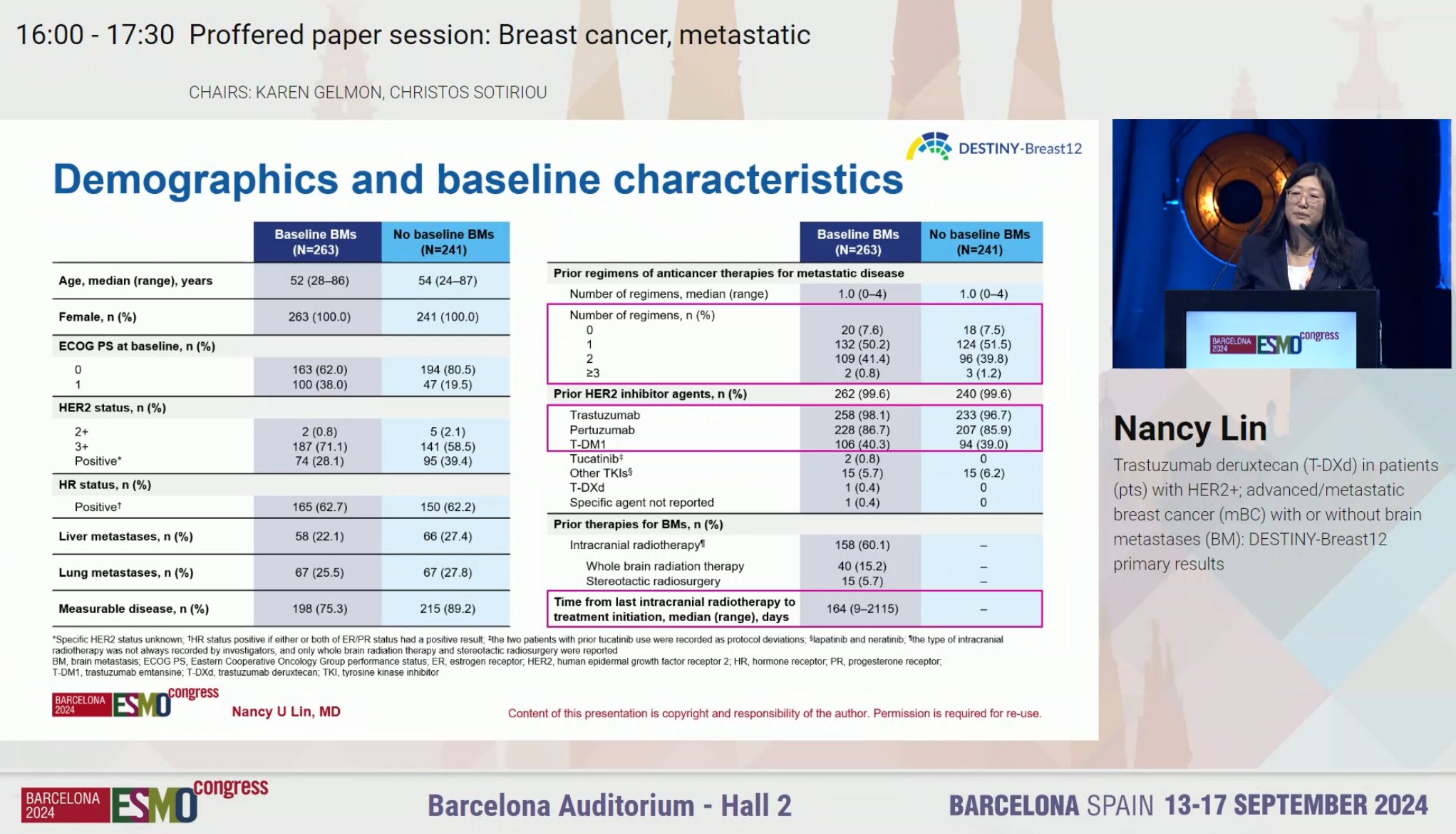
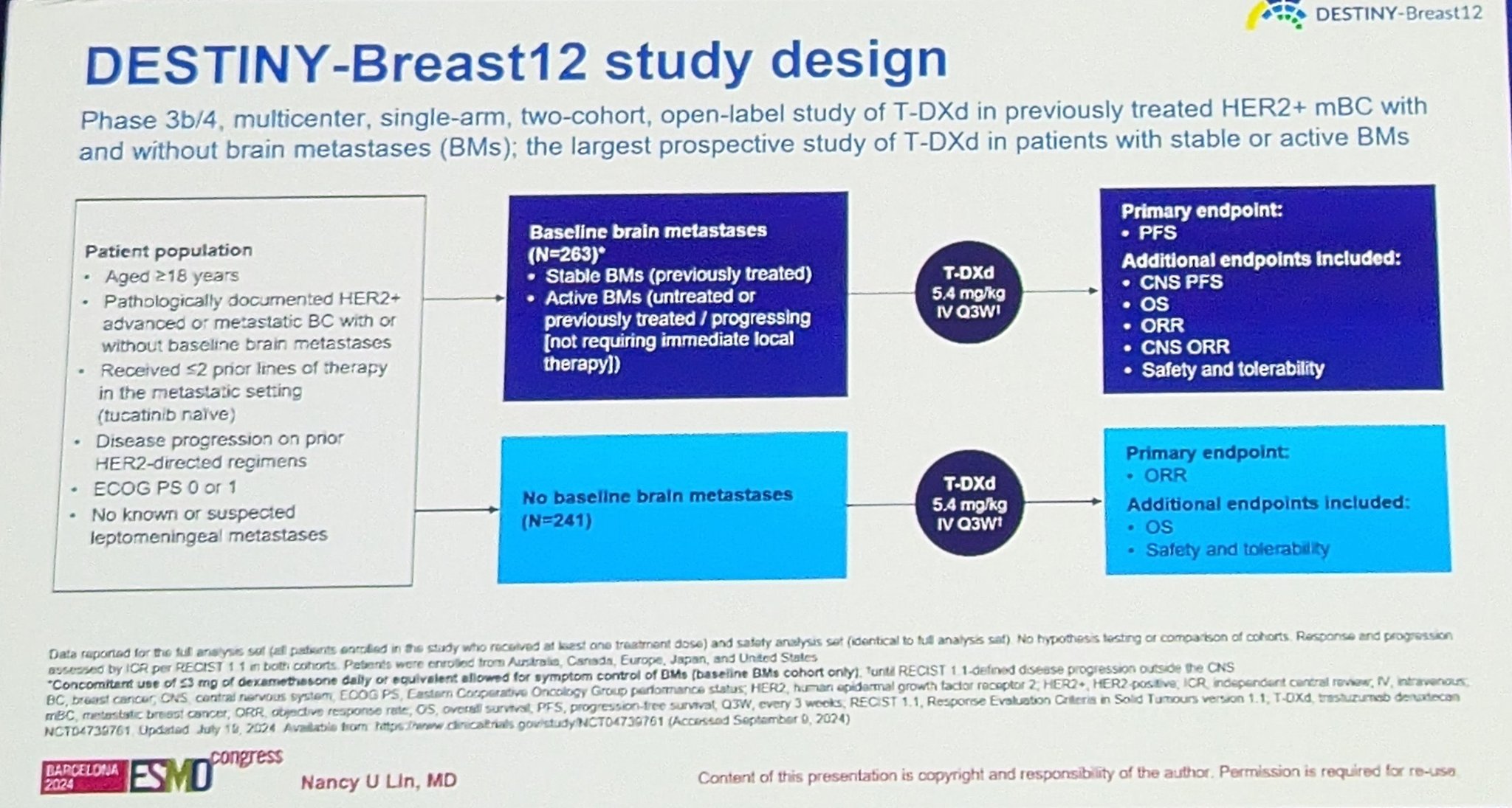
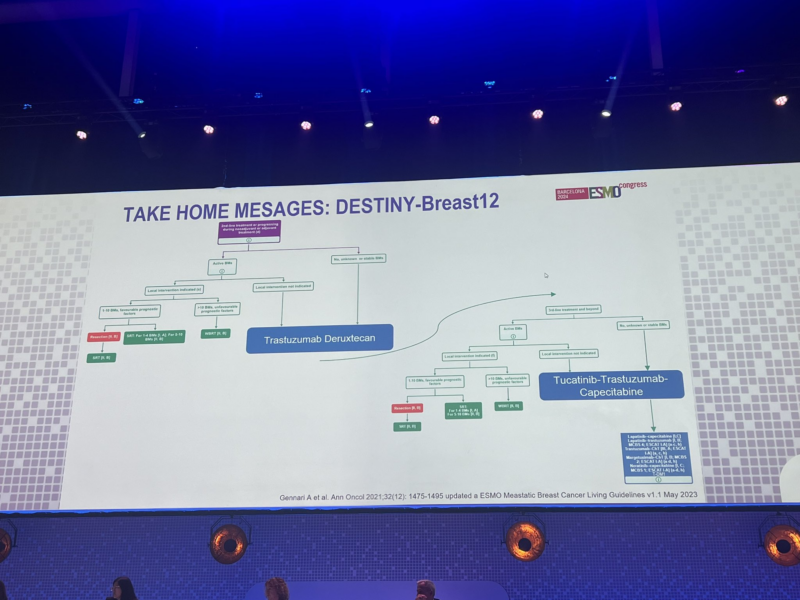
” ‘In my opinion, after today, regardless of brain mets, the standard second line therapy should be T-DXd’ says Cristina Saura of the ESMO Guidelines as she discusses DESTINY-Breast12.”
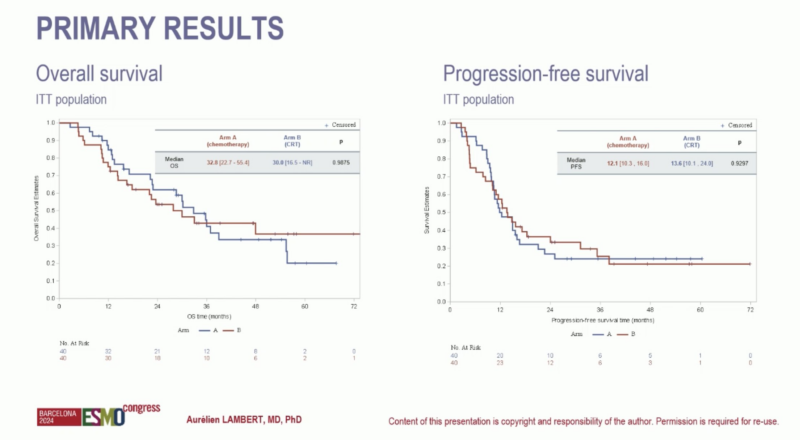
“Radiotherapy does not add to mFOLFIRINOX in borderline resectable Pancreatic Cancer – results for PRODIGE-44 ESMO24.
Good news for patients with PDAC – mFOLFIRINOX had outcomes (mOS 32.8m, 47.9m OS if resected).
Results confirm ESMO guidelines recommendations.”
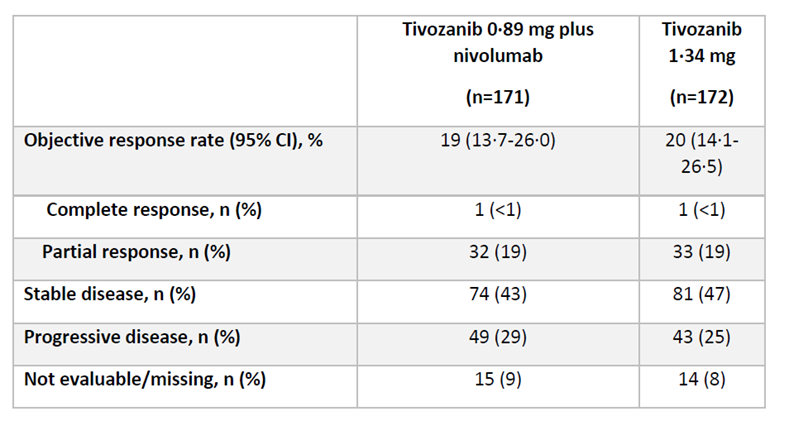
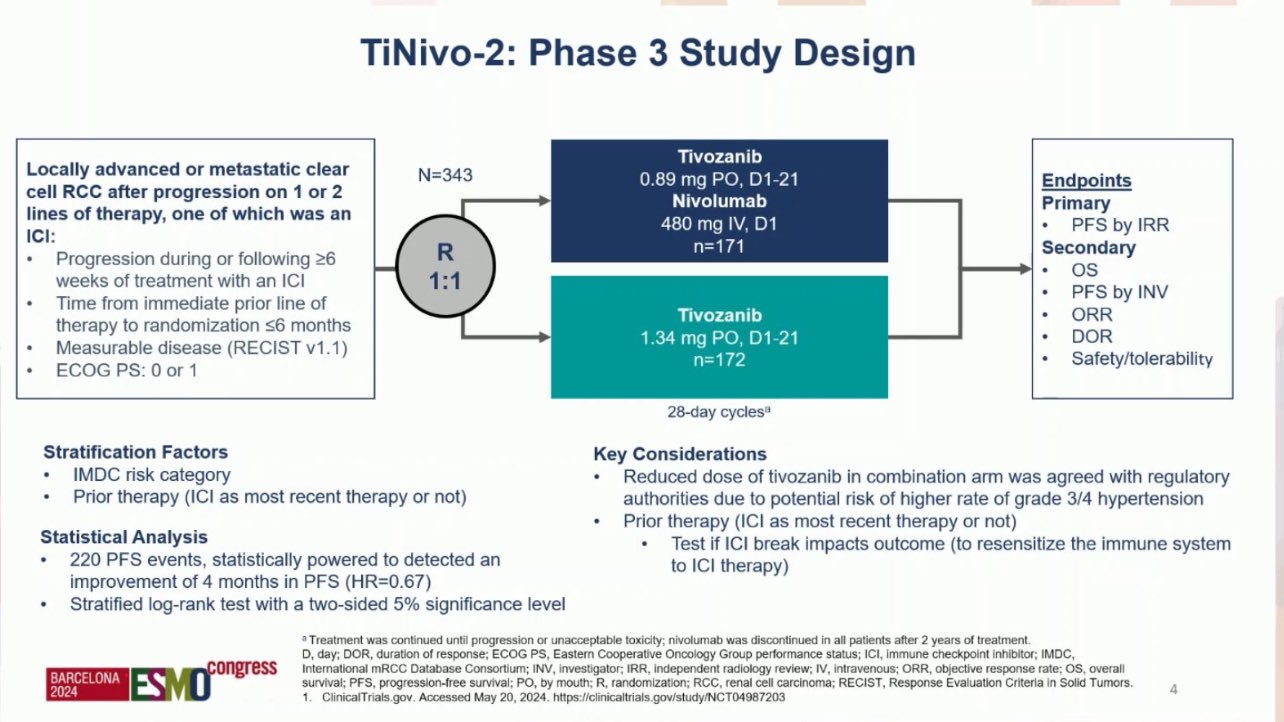
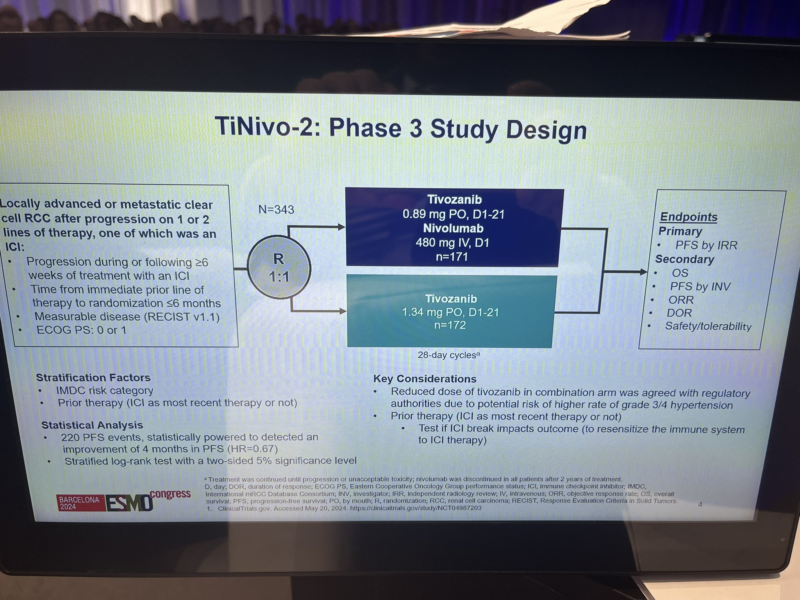
“Results from TiNivo2 are out both ESMO24 and The Lancet! Another phase 3 trial to assess the efficacy of immunotherapy rechallenge, comparing tivozanib + nivolumab vs. tivozanib monotherapy in patients with RCC following an immune checkpoint inhibitor (ICI). (link)
Prior tivozanib trial (TIVO-3) showed an improvement in progression free survival (PFS) with tivozanib compared with sorafenib (HR = 0.55; p=0.028) in the subgroup (26%) that received previous ICI. (link)
CONTACT-03 trial (atezo + cabo to cabo-monotherapy), the first randomized phase 3 evidence of ICI rechallenge in mRCC, revealed negative results, suggesting that ICI rechallenge should be discouraged in pts with mRCC. (link)
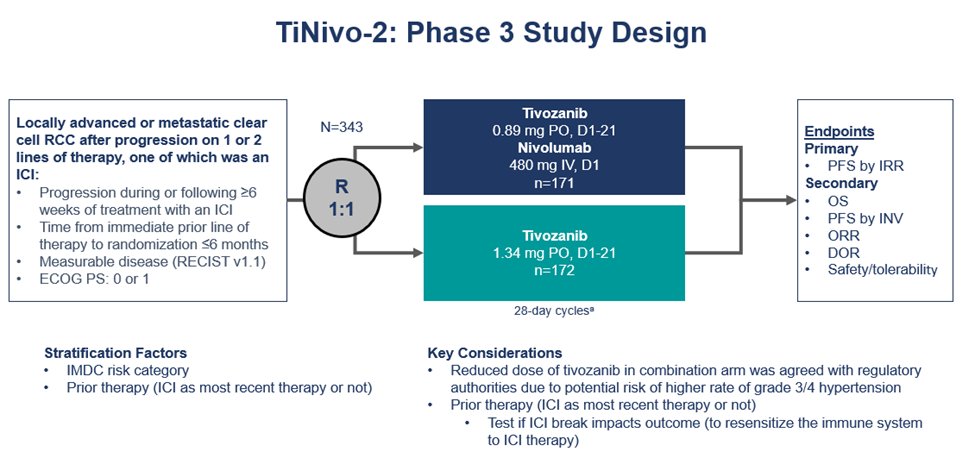
TiNivo-2 is multicenter, randomized, open-label, phase 3 trial
Inclusion criteria were:
- Metastatic ccRCC
- ECOG PS: 0 or 1
- Prior progression on 1-2 regimens (one ICI), following ≥ 6 weeks of ICI
- Time from prior line discontinuation to randomization ≤ 6 months
Patients were randomized 1:1 to tivozanib (0.89 mg/day, orally) + nivolumab (480 mg every 4 weeks, IV) or tivozanib (1.34 mg/day, orally)
- 1ary endpoint: PFS (central review)
- 2ary endpoint: OS/ORR
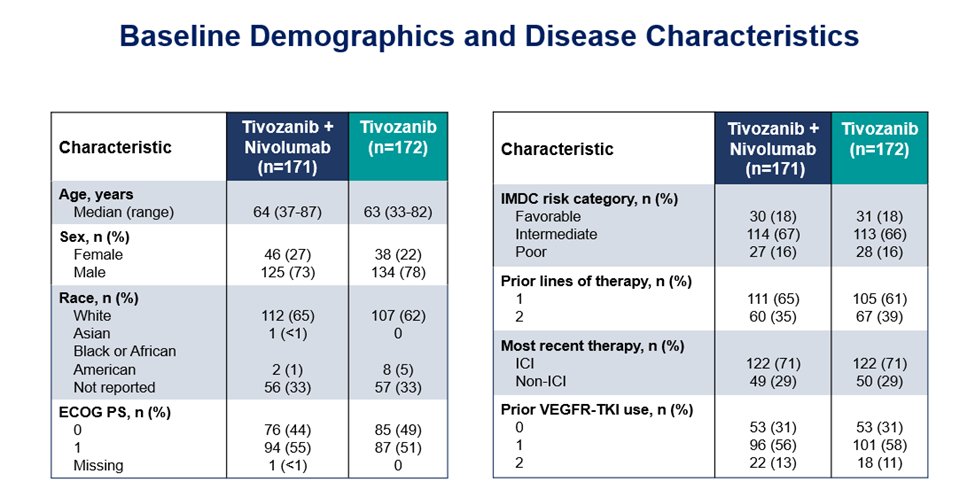
343 pts were assigned to tivozanib-nivolumab (171) or tivozanib monotherapy (172).
Time from randomization to data cutoff was 12.0 months.
Baseline characteristics:
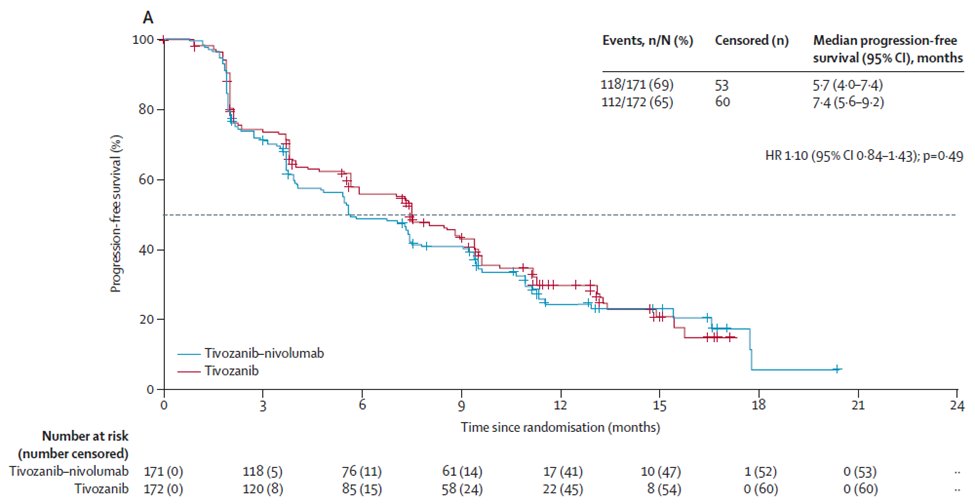
No PFS benefit was observed with Tivo+Nivo vs Tivo only
At 12 months of follow-up, PFS was 5.7 months (95% CI 4.0–7.4) in the Tivo+Nivo group and 7.4 months (5.6–9.2) in
Tivo-monotherapy group (HR = 1.10 (95% CI 0.84–1.43; p=0.49)).
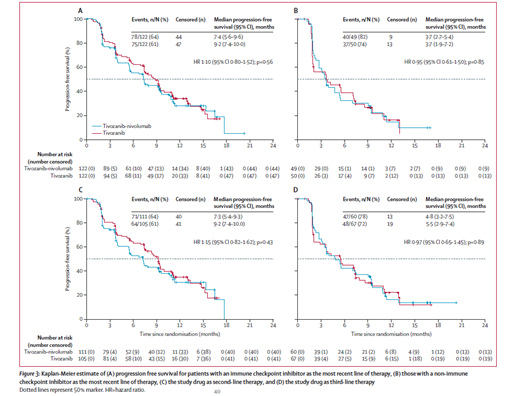
Predefined analyzes per strata were consistent with the primary analysis, whether patients had received an ICI as part of their most recent therapy or not, and whether the study drug was 2nd or 3rdline therapy.
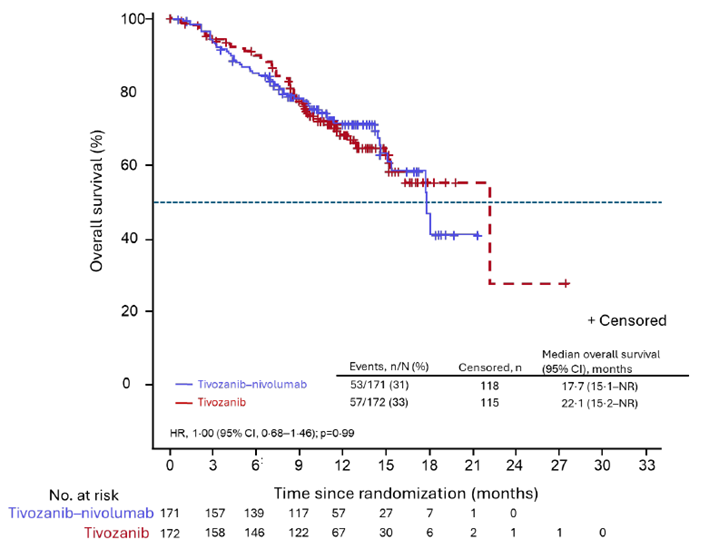
Median OS: 17.7 m (95% CI 15.1–not reached [NR]) in the Tivo+Nivo group vs. 22.1 m (15.2–NR) in the Tivo-monotherapy group (HR:1.0).
ORR: 19% (95% CI 13.7-26.0) in the Tivo+Nivo group vs. 20% (14.1–26.5) in the Tivo-monotherapy group.
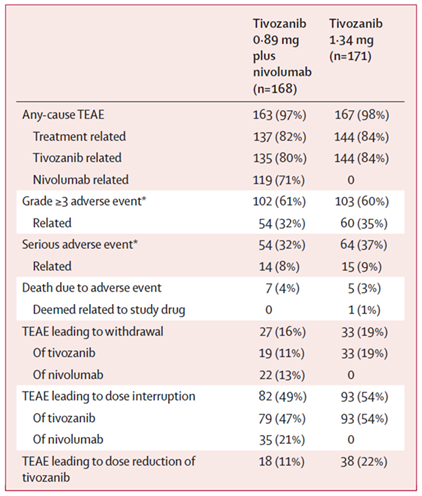
Grade 3+ adverse events (AEs) were ~60% in both treatment arms.
Most common grade 3+ AEs was hypertension, reported equally in both groups
at 75 (22%).
AEs led to withdrawal in 16% and 19% of pts on Tivo+Nivo and Tivo-montherapy, respectively.
In conclusion, the addition of PD-1 inhibitor nivolumab to tivozanib did not result in improved clinical outcomes in patients with mRCC whose disease progressed on or after prior ICI treatment. This trial confirms and expands key conclusions from CONTACT-03 and suggests that ICI rechallenge should be generally discouraged regardless of treatment sequence.
Huge thanks to all the investigators who made this trial possible, to AVEO Oncology, the sponsor, and mostly to our patients and their families, to whom we dedicate all our efforts!”
“Excellent discussion on novel targets in bladder cancer and kidney cancer by Jonathan Rosenberg in ESMO24
New Her-2 targeting ADC and a new HIF-2 inhibitor.”
“Manipulating the microbiome has huge potential in oncology and the field is just opening up. Terrific educational session and special symposium.”
“Important to promote academic trials in rare thoracic conditions. Jordi Remon presents data on PECATI trial: Lenvatinib + Pembrolizumab in metastatic B3-thymoma or thymic carcinoma. 5-months PFS rate, 88%, dose efficacy relationship, and ocasional challenging tox.”
“Congrats Piotr Rutkowski and team for presenting their investigator initiated AXAGIST study on axitinib and avelumab in refractory GISTs. Disease control rate of up to 70% in this hard to treat group!”
“Terrific ending for ESMO24 day one, getting to connect, chat, hug, celebrate with some of the kindest and strongest voices in oncology— thank you OncoDaily for one more superb party!”
“Matteo Lambertini delivered a stellar presentation on fertility preservation and beyond in female patients w cancer at ESMO24.
He highlighted that survivorship is a critical component of cancer care and emphasized the importance of building a well-functioning onco-fertility unit.”

“Distinct clinical outcomes and biological features of specific KRAS mutants in human pancreatic cancer. (link)
KRASG12R tumors are associated with decreased distant recurrence and improved survival as compared to KRASG12D.”

“DESTINY breast 12
- 12 month PFS overall, similiar w stable and active brain mets 62.9% & 59.6%
- median PFS = 17.3 mon
- CNS ORR = 71.7%
- 12-month OS was maintained in patients with BMs (90.3%) and without BMs (90.6%).”
“Kicking off ESMO24 with Toni Choueiri presenting data of the ph3 TiNivo-2 study in patients with mRCC kidney cancer. Adding nivolumab to tivozanib did not improve outcomes in patients with prior ICI exposure.” (link)
“So thrilled to be at ESMO24—my first ESMO! Stopped by the ASCO booth just in time to meet this new ASCO member from Sri Lanka. Welcome! I hope to see many of you while here!”

“Tanja Spanic kicks off the patient advocates reception at ESMO24.
Don’t forget to check out the great patient advocacy track at the meeting.”
“Targeting KRAS in NSCLC
Packed session today on this hot topic:
- Current status of KRAS G12Ci Natasha Leighl
- KRAS Non G12C Collin Lindsay
- Resistance Mark Awad
- KRAS/IO combos Jarushka Naidoo
With great questions from KRAS Kickers.”
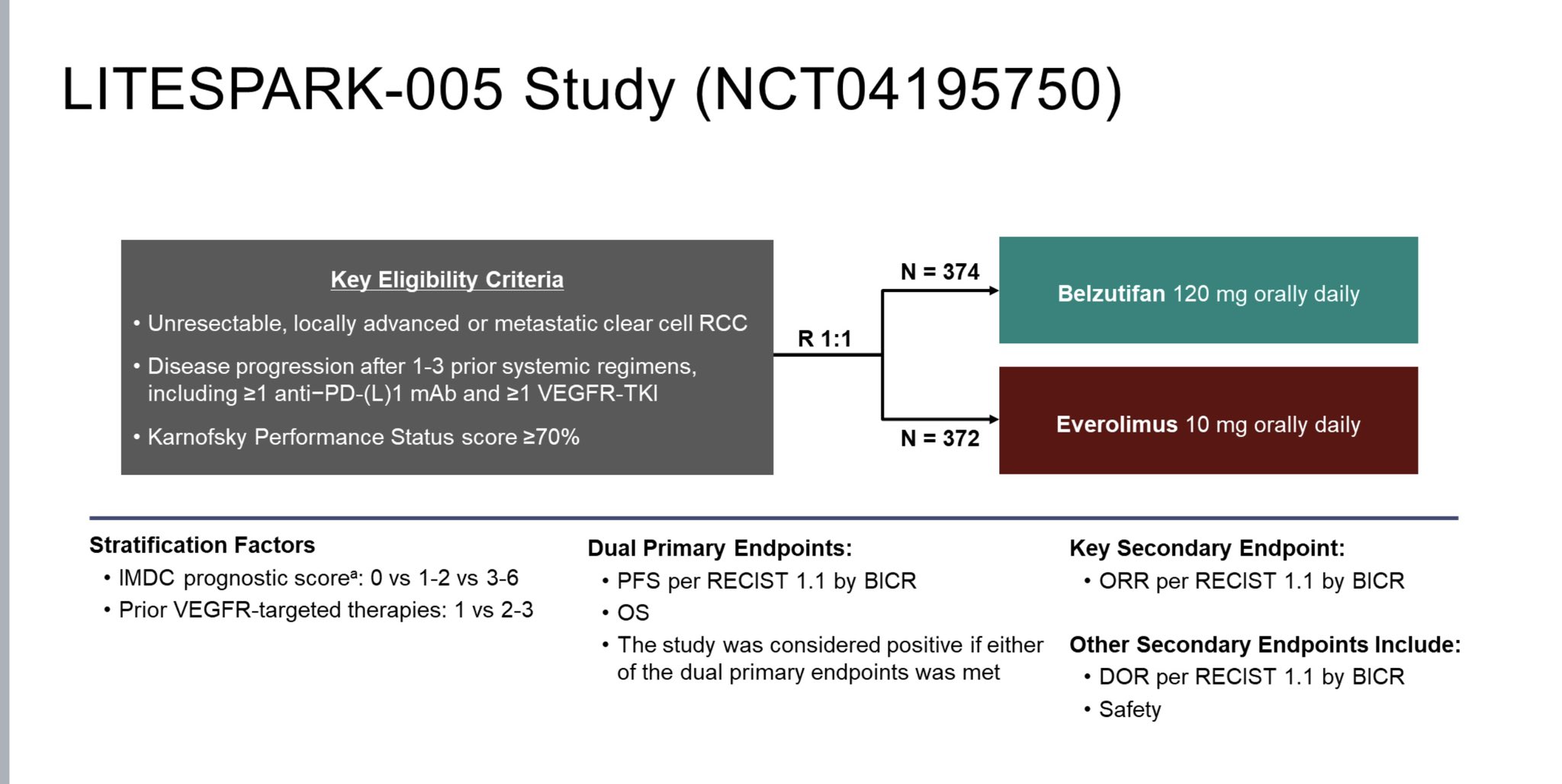
“Belzutifan vs everolimus showed RR and PFS but not significant OS ESMO2024 . Despite this lack of OS belzutifan looks as good as sequencing other VEGF TKI therapy, but with a nice toxicity signal. The ESMO guidelines group gave it a strong recommendation especially 3rd line.”
“Our MD Anderson Cancer Center experts are at ESMO24, where they are sharing groundbreaking research and the latest advances in cancer science. Learn more about our presence in the European Society for Medical Oncology Congress.”
“1. TiNivo2: Ph 3, Tivozanib vs Tivo + Nivo in mRCC after 1L IO +/- TKI:
- Tivo + Nivo did not improve outcomes. Re-challenging with ICI (PD1/PDL1 not recommended)
- Tivo is the only TKI that is studied after IO exposure in RCC (previously approved based off PFS only).”
“Heather McArthur presents the Capitello 290 capivasertib or placebo plus paclitaxel in Met TNBC with PIK3CA mutations and all comers. Primary endpoint OS no different. PFS small trend with no subset revelations. More work needed in TNBC for sure.”
“At ESMO24 Barbara Pistilli presents ICARUS-BREAST01, phase II study of patritumab deruxtecan (HER3-DXd) in HR+/HER2- advanced breast cancer.
Clinically meaningful activity in pretreated patients (median 2 prior lines) with ORR 53.5%, and mPFS 9.4 mos.”
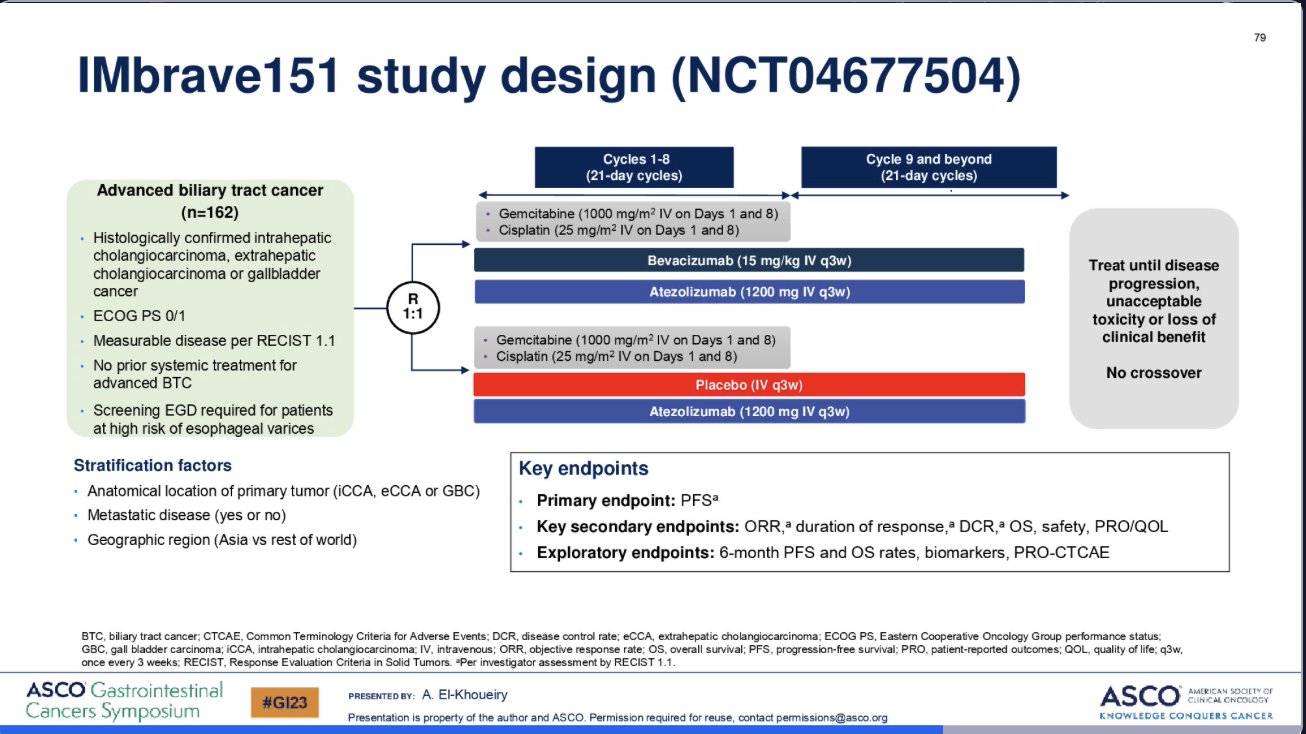
“Atezolizumab with or without Beva in combination with GemCis in biliary tract cancer.
- IMbrave-151, 162 pts
- mOS n.vs 11.4
- mPFS 8.4 vs 7.9 mo
- ORR 25 vs 24%
- Modest benefit with Bev, but OS not yet mature
- KEYNOTE-966 awaited.”
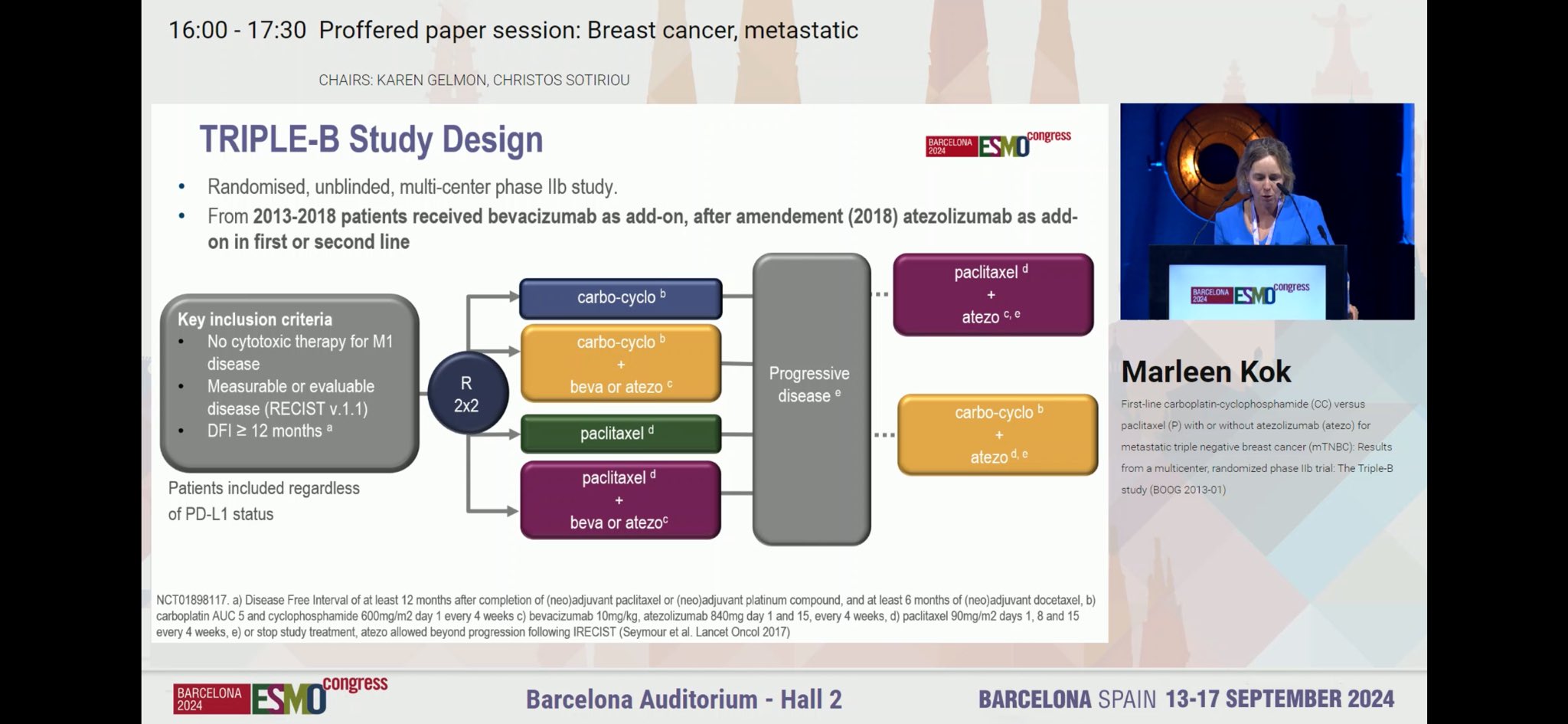
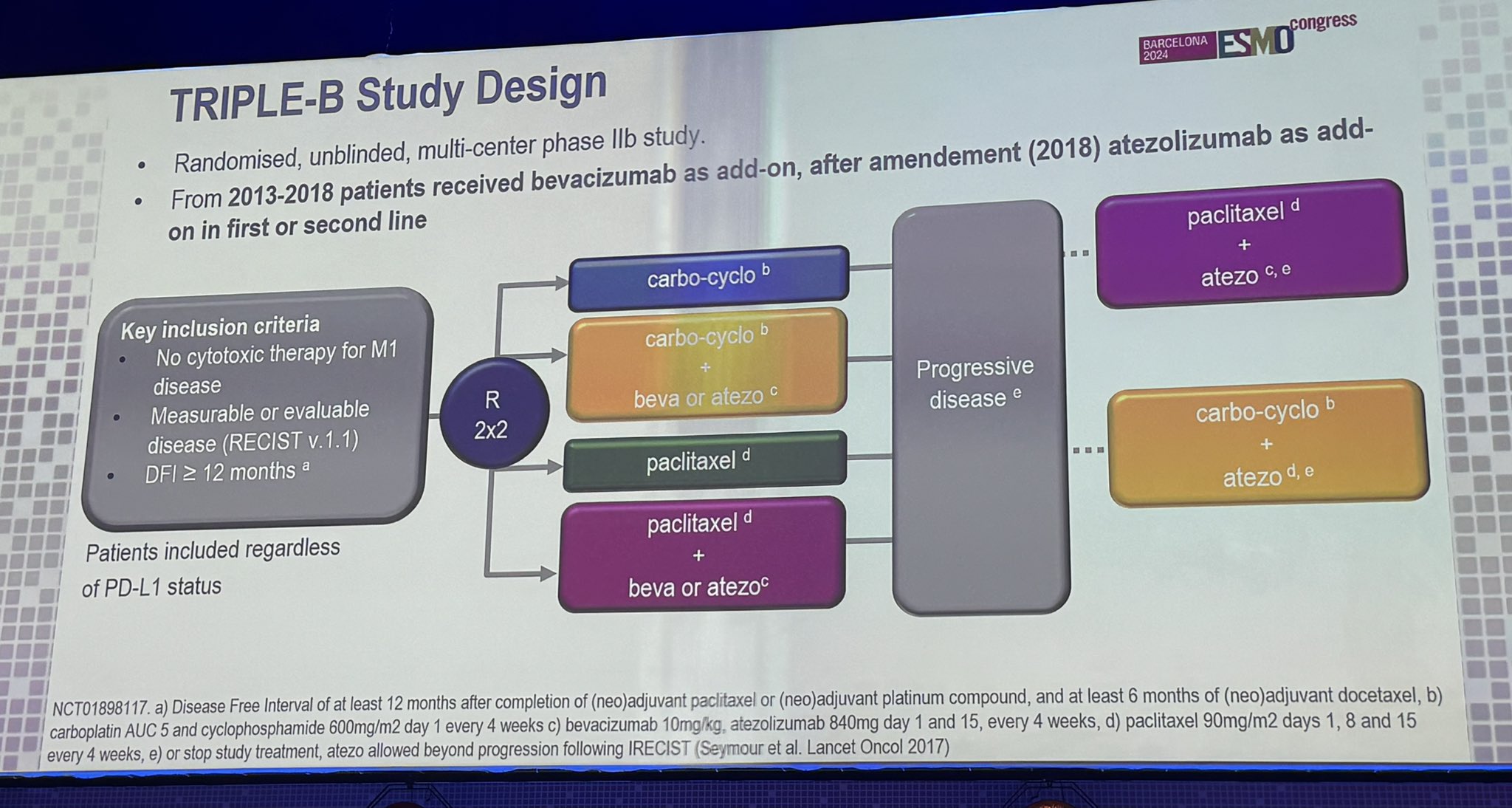
“TRIPLE-B: Carbo/cyclophos vs. paclitaxel by Marleen Kok.
Complicated with add-ons of bev or atezo by when enrolled.
No signif diff of C/C vs. T
With atezo- paclitaxel performs the best.”
“Destiny-Breast12: One of the highlights at ESMO24 presented by Nancy Lin. Practice changing data for mBC patients with HER+ disease and brain metastases. So finally patients with active brain metastases are being included in trials.”
“End of day 1 ESMO24 with Paolo Tarantino GRASP Philippe Aftimos MD and many friends from The Multinational Association of Supportive Care in Cancer (MASCC) and Alejandro R. cc OncoDaily.”
“ESMO – YO Mentorship Session.
Great opportunity to sit down and talk with young oncologists all over the world interested in a career in clinical trial design and conduct, with the expert advise and counsel of a field giant such as Giuseppe Curigliano.
Thank you ESMO – European Society for Medical Oncology for this amazing opportunity.”
“Can’t wait to see all photos of the OncoDaily beach party!
Thanks for having us, such a great network event during ESMO24 .
What has climate change to do with Oncology?
Do not miss Dr. Amy Booth from University of Oxford tomorrow Saturday at 10am Sala Tarragona!”
More posts featuring ESMO24 on oncodaily.com