The WCLC24 is taking place at the San Diego Convention Center from September 7 to 10, 2024.
The WCLC 24 is organised by the International Association for the Study of Lung Cancer (IASLC). This major conference is gathering leading experts, researchers, and oncologists to present and discuss the latest advancements in lung cancer research, treatment, and personalized therapies, fostering collaborative efforts towards overcoming the challenges of lung cancer.
Chul Kim, Associate Professor at Georgetown University, shared some insights from the conference.
Exploratory analysis from MARIPOSA
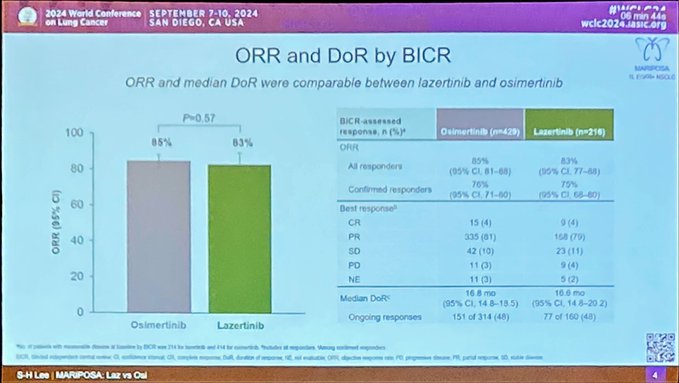
OA02.05 – Lazertinib vs Osimertinib in 1L EGFR+ aNSCLC: Exploratory analysis from MARIPOSA
Comparable efficacy between lazertinib and os including ORR, PFS, OS.
Some differences in AE profiles.
Numerically less QT prolongation and cardiomyopathy with lazertinib.
Important update from MARIPOSA
OA02.03 – Amivantamab/lazertinib vs. osi in 1st line EGFR+ advanced NSCLC: Longer f/u of MARIPOSA by Shirish Gadgeel.
- mOS: NE with ami/laz vs. 37.3 months with osi (HR 0.77)
- 61% alive at 3 years with ami/laz vs. 53% with osi
Important update from MARIPOSA.

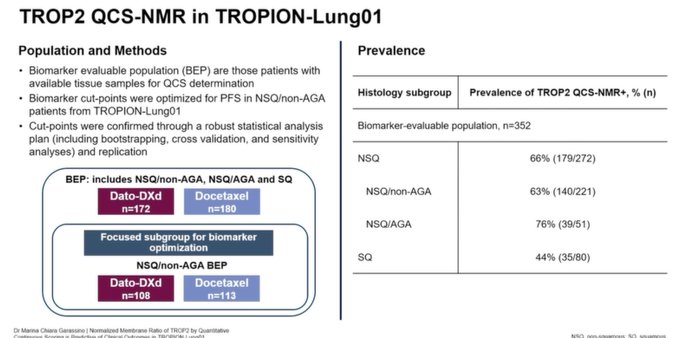
Trop-2 normalized membrane ratio (NMR), measured by quantitative continuous scoring (QCS) in TROPION-Lung01.
Improved ORR and PFS in Trop-2 QCS-NMR positive vs. Trop-2 QCS-NMR negative groups, both in overall biomarker-evaluable population and in NSQ/non-AGA patients.
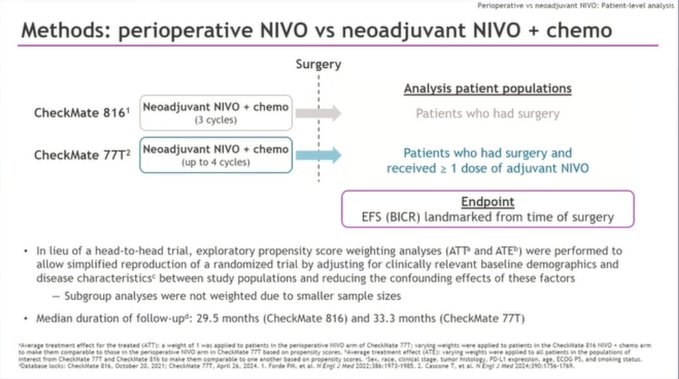
Exploratory analysis of CheckMate 77T vs. CheckMate 816 using propensity scoring weighting analysis.
Landmark EFS: HR 0.61 favoring periop approach.
- In the absence of randomized trials, data is helpful, but a few caveats
- From CheckMate 77T, pts who received at least one dose of adj nivo (some selection here)
- Note more pronounced benefit in pts with no pCR and PD-L1 negative
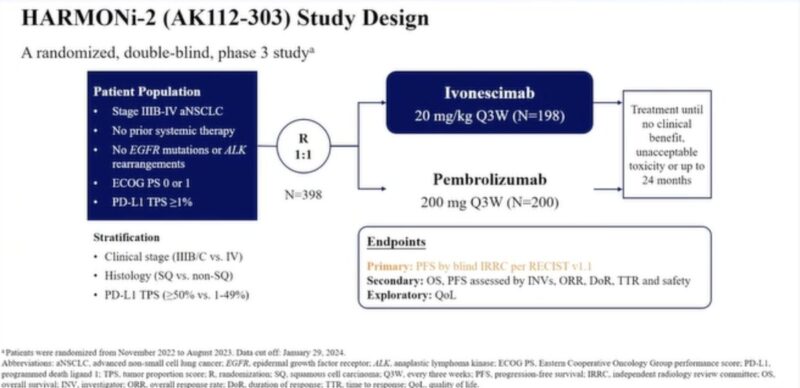
HARMONI-2: Phase III trial of ivonescimab vs. pembro as 1st treatment for PD-L1+ aNSCLC
mPFS: 11.14 vs. 5.82 mo (HR 0.51) favoring ivonescimab.
Benefit seen across subgroups (PD-L1 strata, histology)
ORR: 50.0% vs. 38.5%
Key Questions:
- Is pembrolizumab alone appropriate for PD-L1 1–49%?
- Does this trial need validation in other regions globally?
For more updates on WCLC24, visit oncodaily.com.


