At ASCO 2025, Dr. Emily K. Slotkin and colleagues from Memorial Sloan Kettering Cancer Center (MSKCC) presented findings from a retrospective cohort study exploring the activity of trastuzumab deruxtecan (T-DXd) in patients with relapsed or refractory desmoplastic small round cell tumor (DSRCT). Given the aggressive nature and poor prognosis of DSRCT, these results offer a potentially meaningful therapeutic advance for a disease where treatment options remain limited.
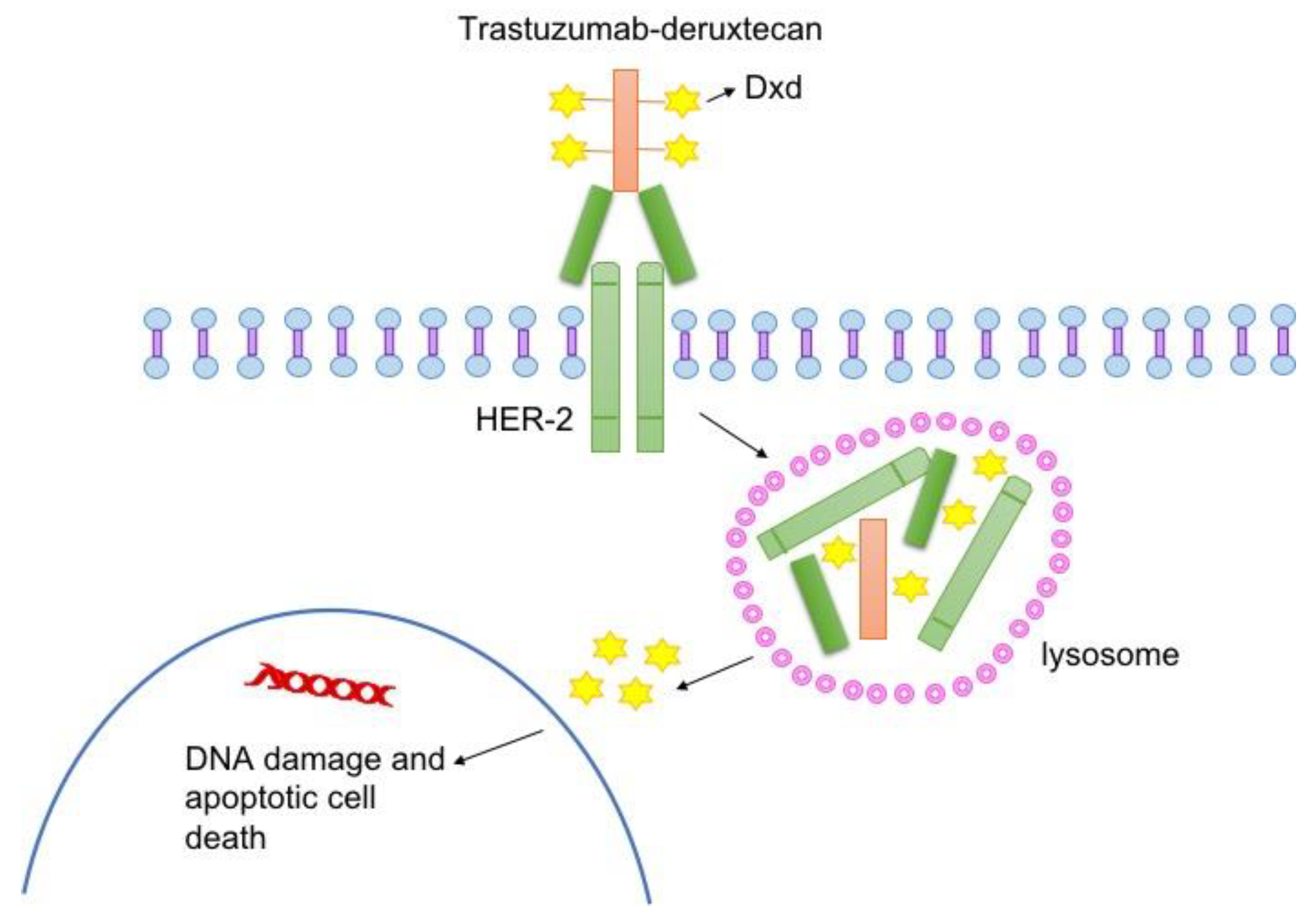
Background
DSRCT is a rare, highly aggressive sarcoma typically affecting adolescents and young adults. Despite intensive, multimodal regimens including chemotherapy, surgery, and radiation, outcomes remain dismal, with most patients eventually experiencing disease progression.
Preclinical studies identified HER2 (human epidermal growth factor receptor 2) as a potential target in DSRCT due to both pathway activation and surface expression. Based on these findings, the MSKCC team initiated HER2-specific assessments in tumor samples from their DSRCT cohort and subsequently administered off-label T-DXd, a HER2-directed antibody-drug conjugate (ADC), to a subset of patients with relapsed/refractory disease.
Methods
The study incorporated a multi-layered approach:
-
Tissue-level HER2 analysis was performed on a tumor microarray (TMA) of 52 unique DSRCT samples, using two immunohistochemistry (IHC) assays—4B5 and CB11.
-
RNA sequencing (RNAseq) was conducted on 61 DSRCT tumor samples to assess HER2 gene expression relative to a broader cohort of 346 pediatric and young adult solid tumors treated at MSKCC.
-
Sixteen patients with relapsed or refractory DSRCT were identified from MSK’s institutional biobanking and genomic profiling protocol. They underwent HER2 testing and received off-label T-DXd therapy, with outcomes monitored retrospectively.
Results
HER2 Expression Analyses
Assessment of HER2 expression in desmoplastic small round cell tumor (DSRCT) revealed striking differences depending on the testing methodology. Using the 4B5 immunohistochemistry (IHC) clone, HER2 expression was minimal—only 3 out of 52 cases showed more than 10% membranous staining, with composite IHC scores ranging from 11 to 15, indicating low-level reactivity.
In contrast, when HER2 was evaluated using the CB11 IHC clone, substantially higher HER2 expression was detected. Thirty-eight of 52 cases (73%) exceeded the 10% staining threshold, with composite scores ranging from 12.5 to 140. This represented a 12.7-fold increase in HER2 reactivity compared to the 4B5 clone, underscoring the variability in HER2 detection based on assay choice.
Further molecular profiling through RNA sequencing (RNAseq) revealed that DSRCT tumors exhibited some of the highest HER2 expression levels across 22 pediatric and young adult tumor types treated at Memorial Sloan Kettering. Among the 346 solid tumor samples analyzed, DSRCT ranked third overall, with only papillary thyroid carcinomaand schwannoma demonstrating higher median HER2 levels. The median HER2 expression in DSRCT was 41.8 transcripts per million (TPM), ranging from 6.3 to 116.3, compared to a median of 9.8 TPM across the broader cohort.
Clinical Outcomes with Trastuzumab Deruxtecan (T-DXd)
All 16 patients with relapsed or refractory DSRCT who received off-label T-DXd experienced clinical benefit, defined as confirmed stable disease or partial response.
-
Treatment was generally well tolerated, with mild side effects including myelosuppression, nausea, and constipation.
-
Importantly, no cases of interstitial lung disease—a known concern with T-DXd—were reported in this cohort.
-
Eight patients (50%) achieved a 30% or greater reduction in tumor size, fulfilling RECIST criteria for partial response, despite all having previously received irinotecan-based therapies.
One particularly notable observation was that treatment response did not appear to correlate with HER2 expression levels by either IHC or RNAseq. Several patients who achieved a partial response had no discernable HER2 expression on IHC, suggesting that T-DXd may exert clinical activity in a biomarker-agnostic manner in DSRCT.
What This Means for Patients
These results provide compelling early evidence that trastuzumab deruxtecan (T-DXd) may offer meaningful clinical activity in patients with DSRCT, even in the absence of robust HER2 protein or gene expression. The absence of severe toxicity and the observation of partial responses in 50% of heavily pretreated patients point to T-DXd as a potential new treatment option in this ultra-rare, high-need population.
Given these findings, a biomarker-agnostic clinical trial of T-DXd in DSRCT is now planned through the Children’s Oncology Group, representing a critical next step in validating this approach.


