At the ASCO Congress 2025, Dr. Rachel O. Abelman and colleagues from Massachusetts General Hospital and Dana-Farber Cancer Institute presented the first clinical results from Arm A2 of the NeoSTAR trial, evaluating the combination of sacituzumab govitecan (SG) and pembrolizumab (P) as neoadjuvant therapy in patients with early-stage triple-negative breast cancer (TNBC). This marks the first study to assess SG/P in the neoadjuvant setting for early TNBC, expanding on previous findings from SG monotherapy (Arm A1).
What Is the NeoSTAR Study?
The NeoSTAR trial (NCT04230109) is a prospective, multi-arm, phase II study evaluating novel neoadjuvant regimens in treatment-naïve early-stage TNBC (defined as tumor size ≥2 cm or node-positive disease). Arm A2 specifically investigates the efficacy and tolerability of sacituzumab govitecan, a TROP-2–targeting antibody-drug conjugate, in combination with pembrolizumab, a PD-1 immune checkpoint inhibitor.
-
Treatment regimen: SG 10 mg/kg on days 1 and 8 + pembrolizumab 200 mg on day 1 of each 21-day cycle, for 4 total cycles.
-
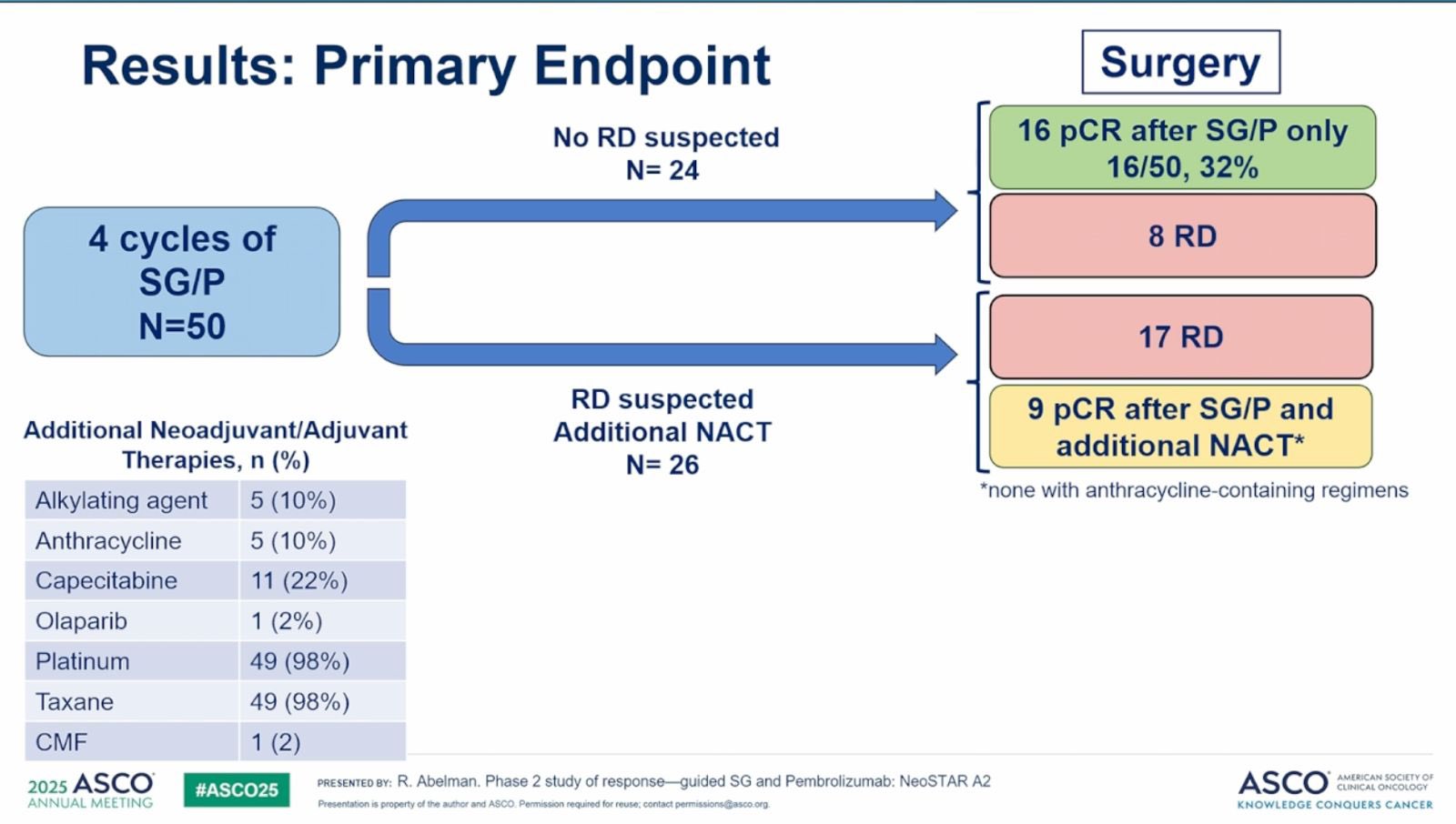
Primary endpoint: Pathologic complete response (pCR) at surgery, defined as no invasive disease in breast or lymph nodes following SG/P without additional chemotherapy.
Secondary endpoints: Radiographic response (RR), need for additional neoadjuvant chemotherapy (ANACT), safety, tolerability, and event-free survival.

Key Findings
Between May 2023 and August 2024, a total of 50 patients with early-stage triple-negative breast cancer (TNBC) were enrolled in Arm A2 of the NeoSTAR trial. The median age of participants was 57 years, with an age range of 23 to 77 years. Most patients (96%) presented with clinical stage II disease, and nearly two-thirds (64%) were node-negative at the time of diagnosis.
The combination regimen of sacituzumab govitecan (SG) and pembrolizumab (P) was generally well tolerated, with 44 out of 50 patients (88%) completing all four planned treatment cycles. Among the six patients who did not complete therapy, five discontinued due to treatment-related toxicities, while one experienced disease progression during the trial regimen.
Following completion of SG and pembrolizumab, patients proceeded to surgery without additional chemotherapy unless residual disease was suspected. In the per-protocol population—those who underwent surgery immediately after SG/P—16 patients (34%) achieved a pathologic complete response (pCR), meaning no residual invasive disease was found in the breast or lymph nodes. The 95% confidence interval (CI) for this outcome was 19.5% to 46.7%.
Radiographic responses were also encouraging: 66% of patients had a measurable clinical response, including 30% with complete radiographic response (CR) and 36% with partial response (PR), based on RECIST v1.1 criteria.
Among the 26 patients who received additional neoadjuvant chemotherapy (ANACT) before surgery—most often due to residual radiographic findings or physician discretion—9 achieved a pCR. This group included:
-
2 patients with biopsy-confirmed residual disease who later had pCR
-
6 patients with negative or inconclusive biopsies
-
1 patient who did not undergo biopsy prior to ANACT
When these ANACT responders were included, the overall pCR rate increased to 50% (25 of 50 patients; 95% CI: 35.5% to 64.5%), indicating the potential value of a sequential treatment approach in some patients.
In a small subset of patients with pathogenic BRCA mutations (n=5), 3 out of 5 (60%) achieved pCR following SG/P alone, and an additional patient achieved pCR after receiving subsequent chemotherapy, suggesting enhanced sensitivity in this biologically defined subgroup.
Safety and Tolerability
The combination of SG and pembrolizumab was generally well tolerated, though 40% of patients experienced grade 3 or higher adverse events. The most common treatment-related side effects (any grade) included:
-
Nausea in 56% of patients
-
Alopecia (hair loss) in 52%
-
Fatigue in 46%
-
Diarrhea in 44%
Dose reductions of sacituzumab govitecan were necessary in 4 patients (8%), reflecting manageable toxicity consistent with prior experience from monotherapy studies in the metastatic setting.
Overall, these findings suggest that the SG/P combination is feasible and active in the neoadjuvant setting for early TNBC, offering a potential chemotherapy-sparing strategy for a subset of patients.
What This Means for Patients
The NeoSTAR Arm A2 findings represent the first prospective data supporting the potential of sacituzumab govitecan + pembrolizumab as a neoadjuvant option in early-stage TNBC. While pCR following SG/P alone was 34%, the total pCR rate increased to 50% when including patients who subsequently received standard chemotherapy, suggesting that a sequential approach may optimize outcomes.
These results provide a strong rationale for continued investigation into the optimal sequencing, duration, and integration of SG/P with other systemic therapies in early TNBC. Updated biomarker and survival analyses, including BRCA and immune correlates, are anticipated at upcoming presentations.
What People Are Saying About the NeoSTAR Trial?
Elisabetta Bonzano, MD/PhD, radiation oncologist and associate professor in Experimental Medicine, shared on X.
“A phase 2 study of response-guided neoadjuvant sacituzumab govitecan and pembrolizumab (SG/P) in patients with early-stage triple-negative breast cancer: Results from the NeoSTAR trial.
Presented by Rachel Abelman, MD”