Al-Ola A Abdallah, Associate Professor and Plasma Cell Disorder Program Director of the Division of HMCT at the University of Kansas Medical Center, shared a post on X:
“Very important topic for future treatment especially for Community oncologists in Rural areas:
Isatuximab Subcutaneous by On-Body Injector (OBI) vs Isatuximab IV Pomalidomide and Dexamethasone in RMM:
Phase III IRAKLIA Study
1st Phase III MM trial to utilize an on-body injector, marking a significant advancement in drug administration methods for this patient population.
531 patients were randomized (OBI, n=263; IV, n=268).
Median follow-up was 12 months.
Patients aged ≥75 years (20.5% OBI vs. 15.3% IV).
Soft-tissue plasmacytoma (28.1% OBI vs. 22.7% IV).
ISS stage III disease (14.1% OBI vs. 10.1% IV).
eGFR <60 mL/min/1.73 m2 (31.6% OBI vs. 23.1% IV).
Refractoriness to lenalidomide as last treatment (69.6% OBI vs. 63.8% IV).
Median treatment duration was similar: OBI, 8.1 months; IV, 8.3 months.
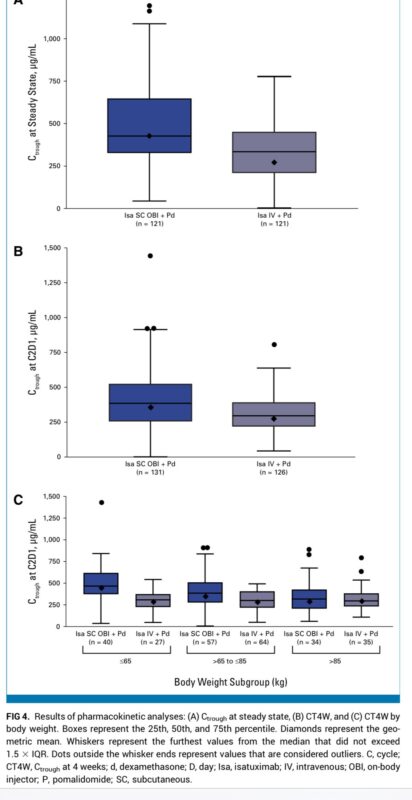
PFS/OS data were immature at the time of this primary analysis.
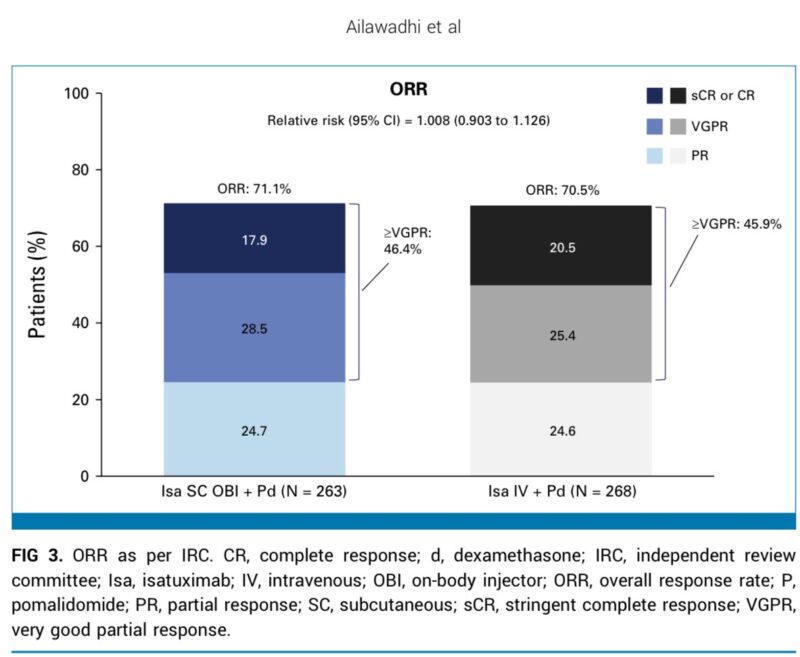
ORR: Isa OBI: 71.1% vs Isa IV: 70.5%
≥VGPR: Isa OBI: 46.4% vs Isa IV: 45.9%
Infusion Reaction Incidence Rate:Isa OBI: 1.5% Vs Isa IV: 25.0%
The majority of IRs for both arms were Grade 1-2. No Grade 4 IRs occurred.
Patient Satisfaction (C5D15 PESQ – satisfied/very satisfied): Isa OBI: 70.0% Vs Isa IV: 53.4%
OBI:Injection site reactions occurred in 0.4% of OBI injections (19 of 5,145 injections). All ISRs were Grade 1-2.
99.9% of injections completed without interruption.
Median OBI injection duration was 13 minutes, with 97.9% completing in ≤20 minutes.
Advantages of Subcutaneous Administration and OBI
The study highlights the broader benefits of SC administration, particularly with the OBI:
Shorter administration duration compared to IV infusions.
Greater patient comfort and satisfaction, as evidenced by the PESQ results.
The OBI uses a ‘thinner, 30-gauge needle versus the 23-25 gauge needle used for SC manual push,” and its “small, hidden, retractable needle… minimizes injury risk and increases comfort.
Reduced chair time and infusion-related resource demands
Improved practice efficiency for healthcare providers (HCPs): “Hands-free SC administration via OBI reduces physical burden and allows for greater nurse efficiency, freeing up nurse time for other clinical and administrative tasks
Potential for at-home administration
Overall safety was similar between arms.
Grade ≥3 treatment-emergent adverse event incidences were 81.7% (OBI) vs 76.1% (IV)
No unexpected safety signal was observed with Isa OBI.
The most common grade ≥3 nonhematologic AEs were pneumonia (OBI 14.8%, IV 15.5%) and COVID-19 (OBI 2.7%, IV 1.9%).
Grade ≥3 laboratory neutropenia was lower in IV patients (74.4%) than for OBI (84.7%), particularly in patients >85 kg.
No patient had persistent anti-drug antibody response; the majority were transient. Incidence of treatment-emergent ADA response was lower for OBI (4.3%) versus IV (9.1%)”
Title: Isatuximab Subcutaneous by On-Body Injector Versus Isatuximab Intravenous Plus Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma: Phase III IRAKLIA Study
Authors: Sikander Ailawadhi, Ivan Špička, Andrew Spencer, Jin Lu, Albert Oriol, Silvia Ling, Fredrik Schjesvold, Alejandro Berkovits, Marek Hus, Chunrui Li, Meletios-Athanasios Dimopoulos, Péter Rajnics, Sevgi Kalayoğlu Beşışık, Vania Hungria, Maria del Rosario Custidiano, Gurdeep Parmar, Xavier Leleu, Fei Li, Claudio Cerchione, Cesar Gomez, Tadao Ishida, Maria Victoria Mateos, Tondre T. Buck, Richard LeBlanc, Jiří Minařík, Hartmut Goldschmidt, Rick Zhang, Dorothée Sémiond, Florence Suzan, Maya Stefanova-Urena, Victorine Koch, Philippe Moreau
You can read the Full Article on Journal of Clinical Oncology

More posts featuring Al-Ola A Abdallah.