At the ASCO Congress 2025, Dr. Timothy A. Yap and colleagues presented the first clinical results of the BNT142-01 trial, a first-in-human study evaluating BNT142, a lipid nanoparticle (LNP)-delivered mRNA therapy encoding an anti-CLDN6/CD3 bispecific antibody (RiboMab02.1). The trial provides early evidence of clinical activity and tolerability, particularly in CLDN6-positive ovarian cancer, and represents the first clinical proof-of-concept for an mRNA-encoded bispecific antibody in oncology.
What Is BNT142?
CLDN6 is an oncofetal cell surface protein that is silenced in normal adult tissues but aberrantly reactivated in various tumors, including ovarian, testicular, non-small cell lung cancer (NSCLC), and other rare malignancies. BNT142 is a novel therapeutic approach that uses mRNA technology to instruct hepatocytes to produce RiboMab02.1, an engineered bispecific antibody targeting CLDN6 on tumor cells and CD3 on T cells, activating a T-cell–mediated anti-tumor immune response.

Trial Design and Methods
BNT142-01 (NCT05262530) is an ongoing Phase I/II, open-label, multicenter trial evaluating weekly intravenous administration of BNT142 in patients with CLDN6-positive solid tumors (defined as ≥10% of cells expressing CLDN6 with at least weak membrane staining). Seven dose levels (DLs) were tested with premedication (e.g., antipyretics, antihistamines, fluids) at physician discretion.
-
Primary endpoints: safety, tolerability, and recommended Phase II dose (RP2D)
-
Secondary/exploratory endpoints: pharmacokinetics, pharmacodynamics, and preliminary efficacy per RECIST 1.1
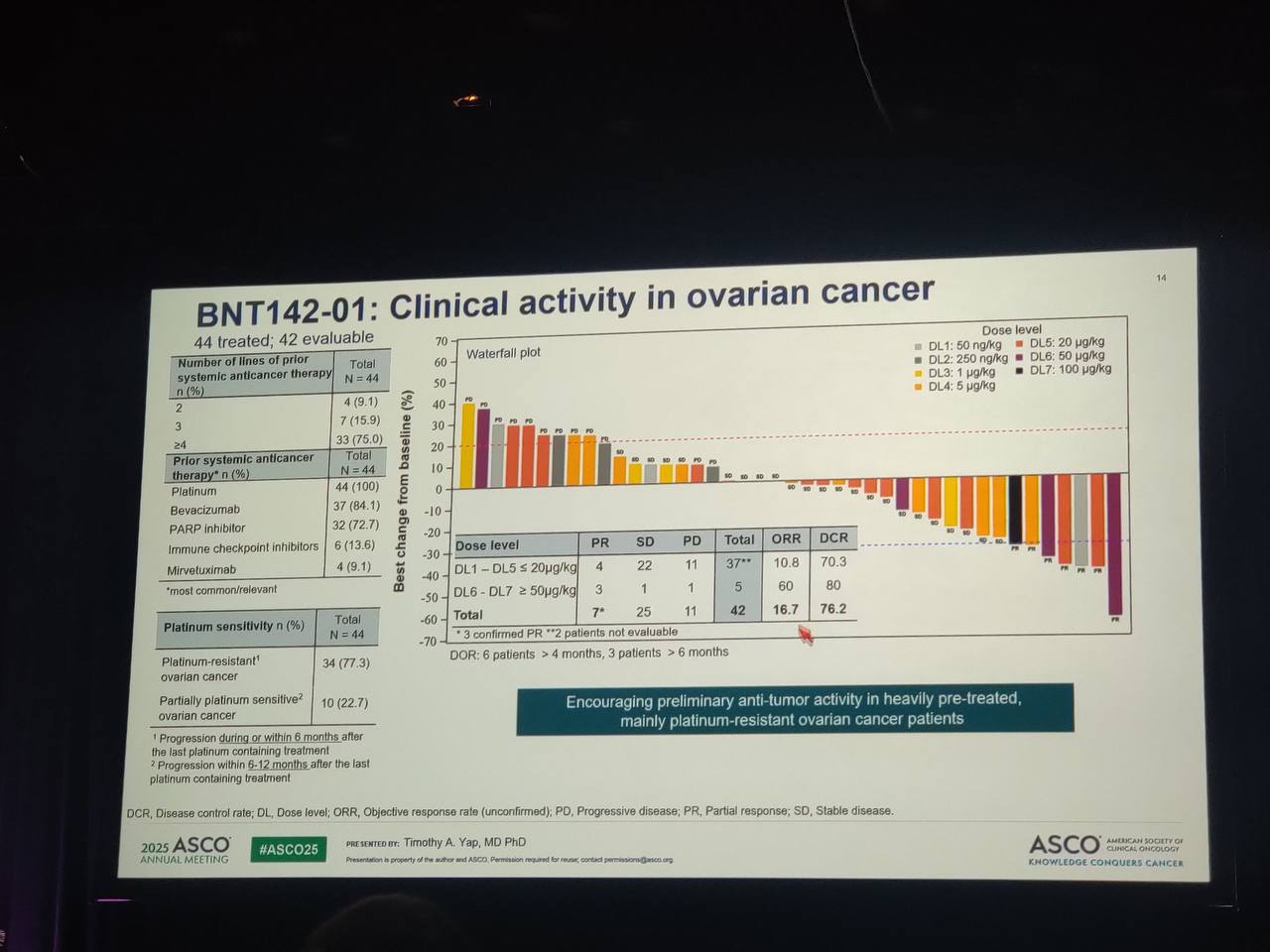
Patients were heavily pretreated, with 71% having received ≥4 prior lines of systemic therapy.
Key Findings
As of December 2, 2024, a total of 65 patients had been enrolled in the BNT142-01 trial and treated across seven escalating dose levels (DLs). The median age of participants was 57 years, ranging from 18 to 79, and the majority (75%) were female. The trial cohort reflected a diverse tumor landscape, with 44 patients diagnosed with ovarian cancer, 10 with testicular cancer, 5 with non-small cell lung cancer (NSCLC), and 6 with various rare tumors. On average, patients received seven doses of BNT142, though this ranged from just one dose to as many as 38.
Safety and Tolerability
The treatment demonstrated a manageable safety profile. Treatment-related adverse events (TRAEs) occurred in 63%of patients, and were predominantly mild to moderate in severity. However, 23% of participants experienced grade 3 or higher TRAEs. The most frequently reported side effects included cytokine release syndrome (CRS), which affected 22% of patients, although only one case reached grade 3 severity. Elevated liver enzymes (AST or ALT) were reported in 19%, with 12% of patients experiencing grade 3 or higher elevations. Other common side effects, such as fever, chills, and fatigue, occurred in approximately 12% of participants, but were mostly low-grade and transient.
Two dose-limiting toxicities (DLTs) were documented during the escalation phase: one patient in DL5 experienced a grade 4 ALT elevation, and another patient in DL6 experienced a fatal grade 5 CRS event. Despite these serious cases, the regimen remained generally tolerable. As a result of TRAEs, 2% of patients required dose reductions, 19% experienced treatment interruptions, and 3% discontinued therapy. Most of these events were associated with elevated liver enzymes or infusion-related reactions.
Translational and Pharmacodynamic Insights
From a translational science perspective, BNT142 induced transient but dose-dependent increases in inflammatory cytokines, indicating immune system engagement. Importantly, the bispecific antibody RiboMab02.1, encoded by the mRNA, was successfully translated in vivo and detected in the serum of patients. Peak levels were observed 24 to 72 hours after dosing, and the amount of circulating antibody increased proportionally with dose level, confirming efficient and controlled delivery via mRNA-LNP technology.
These pharmacodynamic observations provide compelling proof-of-mechanism, demonstrating that BNT142 achieves both its intended biological activity and systemic exposure, critical steps in the clinical development of mRNA-based bispecific therapeutics.
Early Efficacy and Clinical Activity
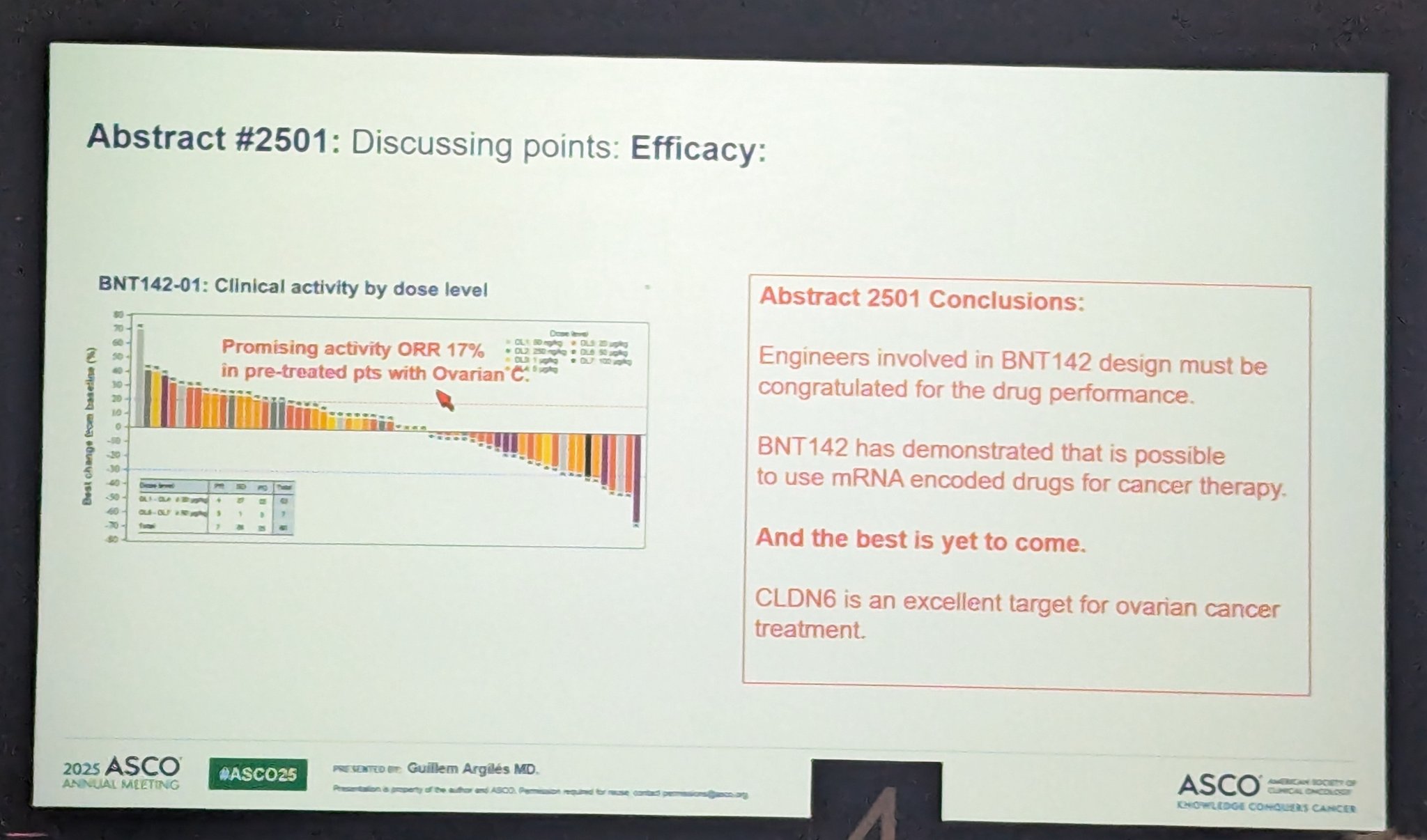
Preliminary data from the BNT142-01 trial demonstrate encouraging signs of clinical activity across all dose levels tested. The overall disease control rate (DCR)—defined as the proportion of patients achieving either a partial response or stable disease—was 58%, indicating meaningful disease stabilization in a heavily pretreated population.
Responses were especially noteworthy in patients with CLDN6-positive ovarian cancer, a subtype traditionally considered resistant to immunotherapy. Among these patients, seven partial responses (PRs) were observed based on RECIST 1.1 criteria, resulting in a DCR of 75%. These findings suggest that higher dose levels of BNT142 may be associated with greater antitumor activity.
Importantly, this early evidence supports the potential of BNT142 to overcome immune resistance in challenging tumor types such as ovarian cancer, potentially broadening the scope of immune-based therapies in solid tumors that have historically shown limited responsiveness.
What People Are Saying About the BNT142-01 Trial?
Kaissa Ouali, Gynecologic Oncologist from Gustave Roussy Cancer Center, Medical Oncologist in the Drug Development Department (DITEP), shared on X.
“mRNA bispecific CD3/CLDN6 BNT142 showed promising preliminary activity in heavily pretreated OC pts -> ORR 17% -> 21% CRS mostly low grade; -> 12% of pts experienced G3+ LFT elevation”
What This Means for the Future
The BNT142-01 trial represents a milestone in mRNA-based immunotherapy—showing that mRNA can be used not only for vaccines but also to generate complex bispecific antibodies in vivo. The promising activity in CLDN6+ ovarian cancer and the manageable safety profile open a new therapeutic avenue for solid tumors traditionally unresponsive to checkpoint inhibitors.
With dose optimization ongoing, future phases of the trial will aim to refine the therapeutic window and potentially expand into combination regimens or earlier lines of treatment.