WCLC (The World Conference on Lung Cancer) 2025 in Barcelona, Spain highlighted how immunotherapy continues to reshape lung cancer care. This year’s meeting showcased important updates from clinical trials, translational research, and real-world studies—covering new checkpoint inhibitors, novel combinations, and biomarker-driven strategies.
Experts and researchers actively shared impressions and insights across LinkedIn and Twitter, sparking vibrant discussions within the global oncology community. In this article, we bring together some of the most interesting community reactions and expert takes on the immunotherapy abstracts presented at WCLC25.
Amol Akhade (Consultant Medical Oncologist at Suyog Cancer Clinics):
” Toripalimab Consolidation in Limited-Stage SCLC – Promising but Needs Caution,
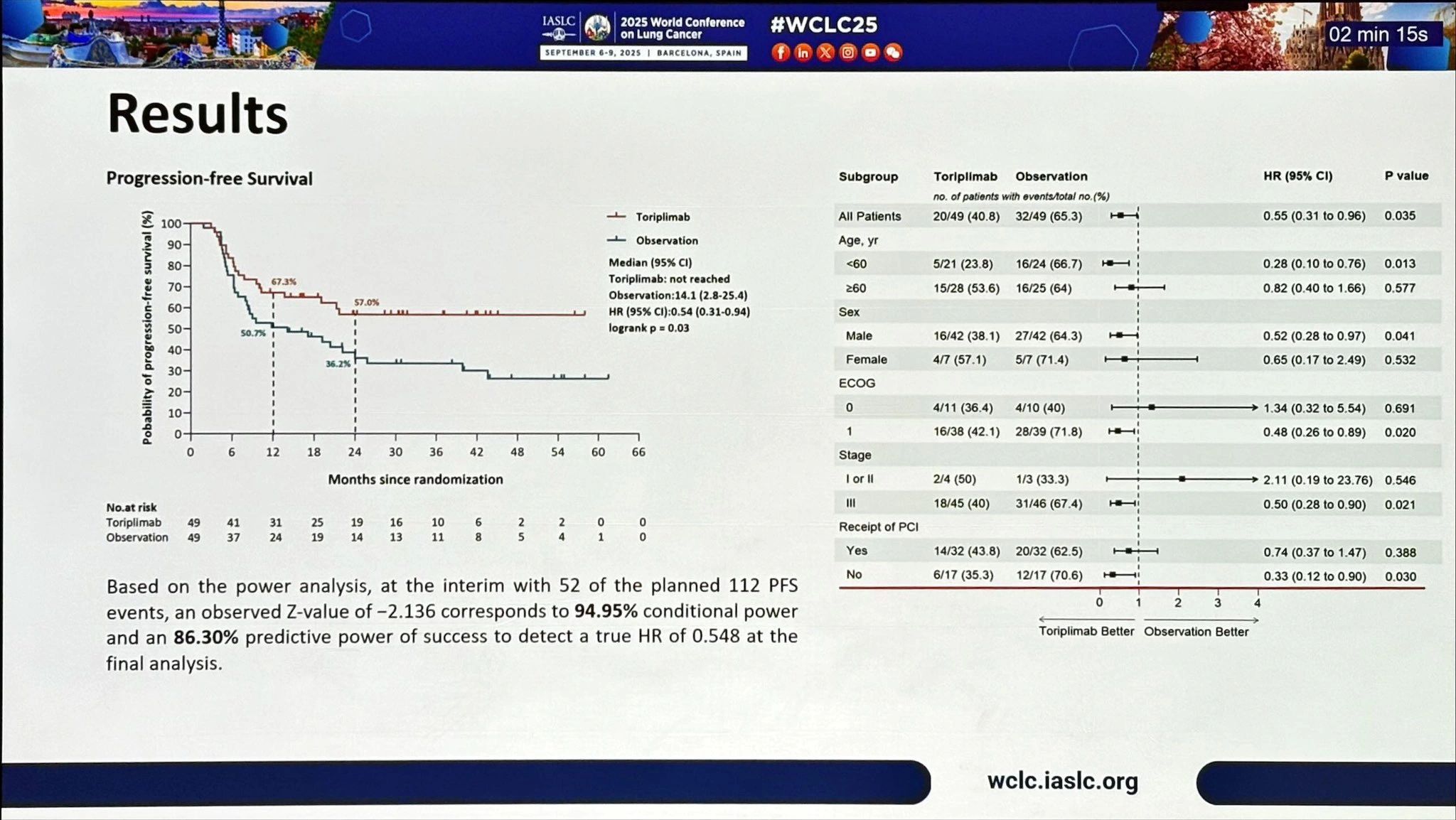
A single-institution, randomized phase II trial (N=98) reported outcomes of toripalimab consolidation following chemoradiation in patients with unresectable, limited-stage SCLC.
Key Findings
- Progression-Free Survival (PFS): Not reached vs 14.1 months (HR 0.54, p=0.03)
- Overall Survival (OS): Not reached vs 30.3 months (HR 0.41, p=0.01)
- Brain Progression: HR 0.47, indicating reduced CNS relapse
- 2-Year OS: ~79% vs 59%
On first impression, these hazard ratios appear even stronger than the landmark ADRIATIC trial (durvalumab, phase III, N=749, global) where:
- PFS: 16.6 vs 9.2 months (HR 0.76)
- OS: 56 vs 33 months (HR 0.70)
- Brain Relapse: HR 0.64
So—is toripalimab the “better” drug? Not so fast.
Critical Appraisal
- Design and Size: Toripalimab: single-center, 98 patients, 6 months of IO, phase II. ADRIATIC: multicenter, 749 patients, 24 months of IO, phase III with OS as co-primary. Small trials often overestimate effect sizes.
- Population: Toripalimab: 100% Asian cohort. ADRIATIC: ~50% Asian, 50% rest of the world → far more representative.
- Endpoints: Toripalimab: PFS as primary endpoint; OS secondary. ADRIATIC: OS and PFS co-primary → more definitive.
- PCI and CNS Protection: Subgroup suggests OS benefit mainly in those not receiving PCI, hinting CNS protection may be a key driver. Raises an interesting debate as PCI use is declining worldwide.
- Follow-up and Durability: OS “not reached” in a 98-patient phase II with short follow-up ≠ definitive durability. Risk of attenuation as curves mature. Broader Perspective
Toripalimab raises an important question: Could 6 months of immunotherapy consolidation be enough, rather than 2 years as in ADRIATIC?
If validated, this would have major cost and access implications in LMICs. But until we see confirmatory phase III data, ADRIATIC with durvalumab remains the standard of care.
Toripalimab data are encouraging, especially regarding CNS relapse reduction. But the small, single-center phase II design means results should be interpreted cautiously. For now, durvalumab consolidation after CCRT remains the global standard. Toripalimab represents an interesting, potentially shorter and more affordable alternative—but only further large-scale studies will confirm whether “less is truly more.”
Tom Powles (Head of Solid Tumor Research at Barts Cancer Institute):
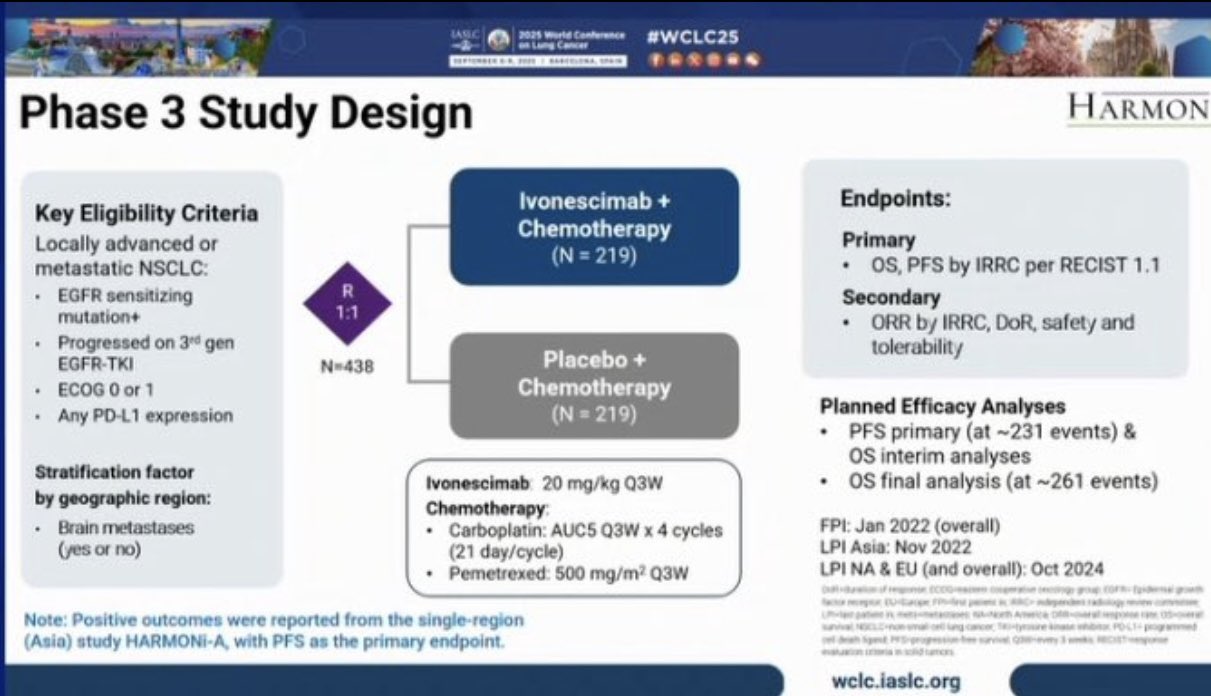
‘VEGF/PD1 bispecifics presented WCLC25 M1 NSCLC. Ivonescimab/chemo vs Placebo/Chem PFS HR 0.52 (0.41-0.66) & OS HR 0.79 (0.61-1.01). 11% in RR. N=430 is small for a R3 and underpowered for significant OS. This class needs investing in combinations in renal and bladder cancer.’
Benjamin Besse (President-Elect of EORTC and Head of Clinical Research at Gustave Roussy):
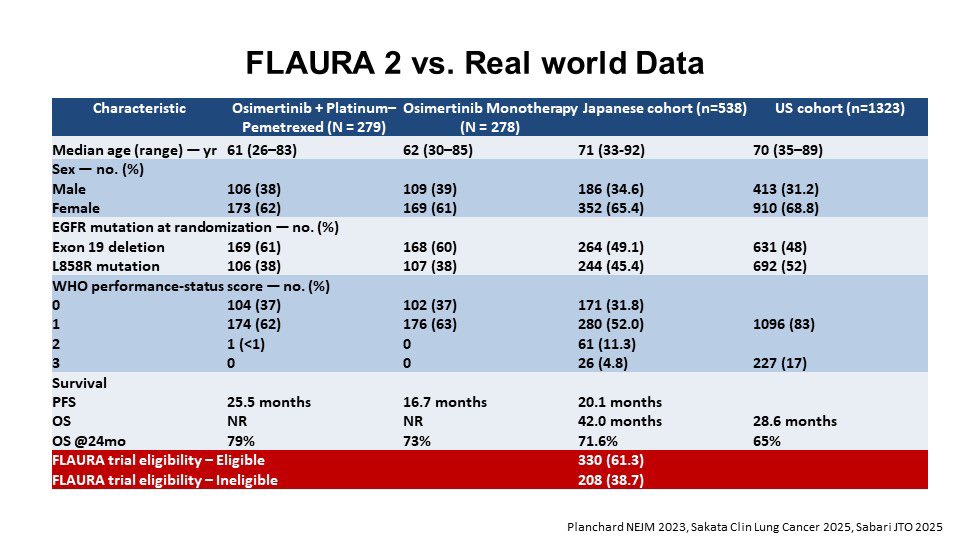
” EGFR update 7 potential options:
- 3rd gen TKI: osimertinib, lazertinib, aumolertinib
- Amivantamab
- Pemetrexed
- Carboplatin Ivonescimab
- Dato-DXd
OS data favor combos upfront—but real-world ≠ trial. In RWD, ~40% of newly diagnosed pts wouldn’t qualify for FLAURA2 WCLC25″
Bartomeu Massuti (Associate Professor at Miguel Hernandez University of Elche):
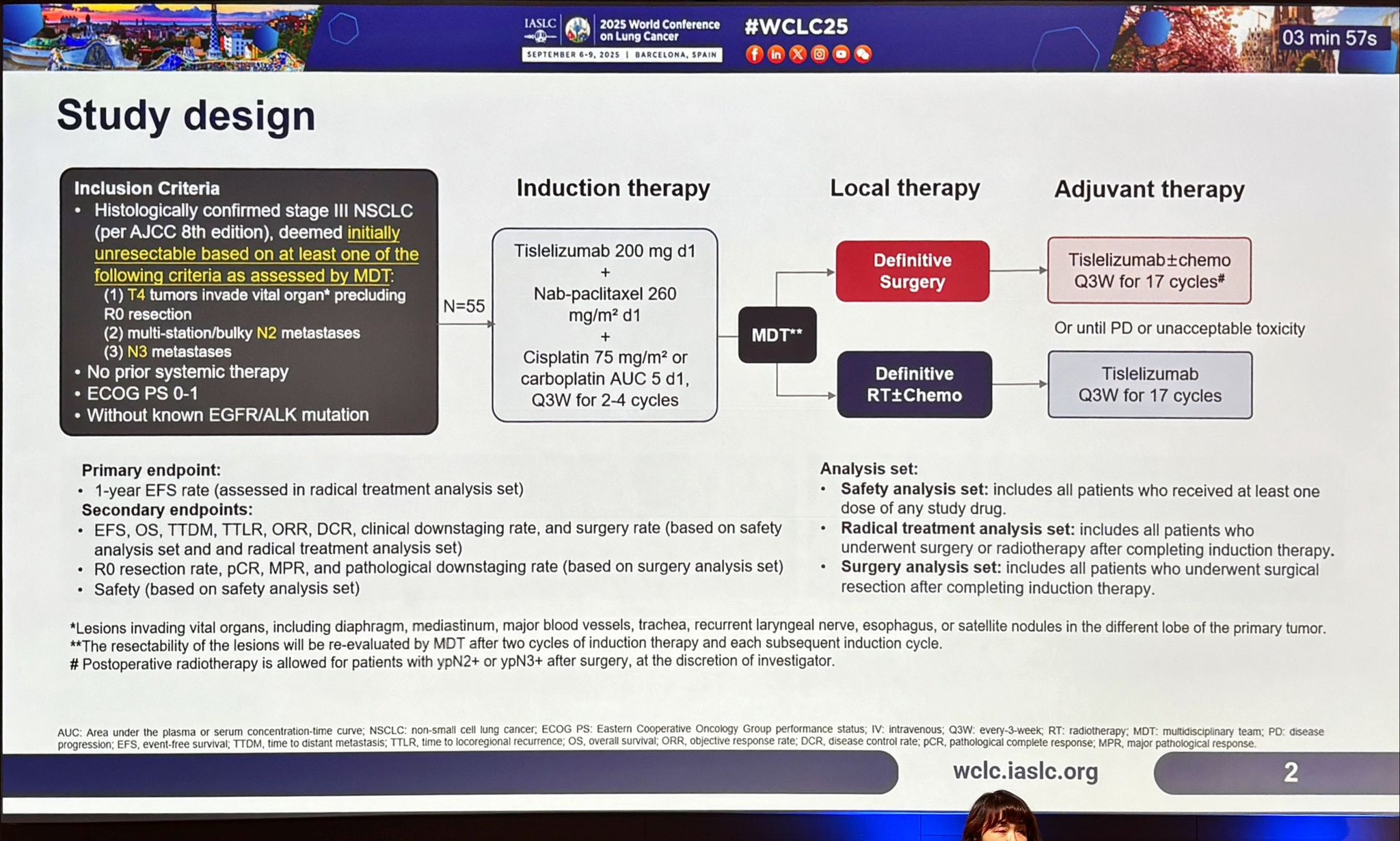
“Upfront chemo-immunotherapy approach is winding the borders for surgery in locally advanced St III NSCLC at WCLC25”
” 76.9% surgery conversion rate and 55% pathological complete response in “considered unresectable” St III after induction Tislelizumab plus Platinum-based chemotherapy: small number of patients but impressive results”
Written by Toma Oganezova, MD, Editor-in-Chief of OncoDaily IO


