The 2025 IASLC World Conference on Lung Cancer is underway in Barcelona, Spain, bringing together the world’s leading experts in lung cancer and thoracic oncology. This annual conference serves as a critical forum for clinicians, researchers, and industry leaders to explore the latest scientific discoveries, innovative treatments, and emerging trends in lung cancer care.
At the WCLC 2025 Presidential Symposium Dr.David Planchard presented the Overall Survival (OS) results from the FLAURA2 trial. This pivotal study demonstrated that adding chemotherapy to osimertinib significantly improves survival outcomes in first-line treatment for EGFR-mutated advanced non-small cell lung cancer (NSCLC), marking a major advancement in patient care. Here are selected expert reactions and highlights you shouldn’t miss.
Our team at OncoDaily has selected a few posts from WCLC 2025 about FLAURA2 Trial:
“It was an honor to present, on behalf of the co-investigators, the planned OS results from FLAURA2 ph III at WCLC25. These results confirm a shift in our first-line practice for our EGFRm pts, marking the first time a study has achieved a significant benefit mOS of 4Y.”
“Dr. David Planchard at WCLC25 with highly anticipated Presidential Plenary presentation with OS results from FLAURA2: first line chemo + osimertinib improves OS from 37.6 to 47.5m, HR 0.77, 4y OS rate 41% to 49%. Benefit seen across subgroups. Strong results – similar to MARIPOSA.
Chemotherapy duration shown here – and fairly high crossover rates are reassuring. More toxicity, yes, but no new signals seen here. Question remains – which regimen for which patient? But a very welcome advance and impressive presentation from David Planchard at WCLC25.”

“FLAURA2 trial: Osi + CT improves PFS & OS ( 23% risk death) vs Osi alone in mEGFR NSCLC. Qx:
– Intensification for all pts? Benefit in some subgroups only (Brain)
– Chemo or ami for intensification?toxicity QoL, relevant for decisions
– Cost
– Is Osi alone no more valid?”

“Just presented at the presidential symposium IASLC WCLC25
The long awaited Overall Survival data from: FLAURA2 trial of Osimertinib + Chemotherapy vs Osimertinib in 1st line Tx for patients with EGFR+ advanced Lung Cancer (NSCLC). Impressive Statistically Significant & Clinically Meaningful Improvement in Overall Survival (OS)
HR: 0.77
mOS: 47.5 vs 37.6 months
No new Safety Signals.”
“WCLC25 Presidential Superb FLAURA2 OS discussion by Daniel Tan, main questions:
– combo may likelihood of long-term response?
– clinical/molec/ctDNA factors relevant
– refinement needs complex integration of factors Extremely elegant slides, I plan to use ”

“In FLAURA2 trial chemotherapy plus Osimertinib increases survival vs Osi single agent with HR 0.77 and median survival 47.5 months and 45% patients alived at 4 years. Is still a role for Osimertinib single agent?”
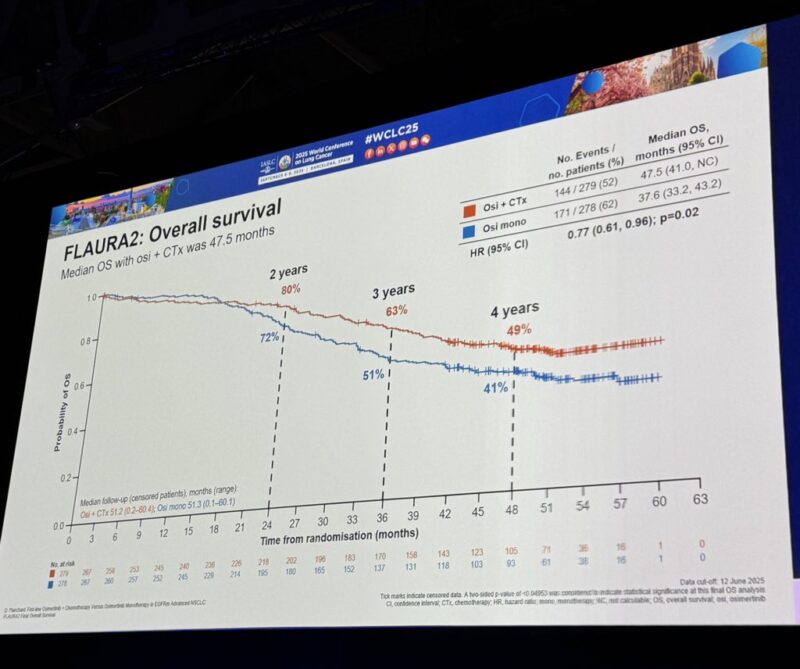
“The key slide from WCLC25 FLAURA2 OS 1L osimertinib + chemo with superior OS vs osi alone
Median OS: 47.5 mo (HR 0.77; 95% CI 0.61–0.96; p=0.02)
“Longest OS ever in a global phase 3 EGFRm NSCLC trial.”
“The new FLAURA2 OS data presented at WCLC25 is exciting – nearly four years of median OS with osimertinib + chemotherapy is remarkable progress for our community!
Alongside the MARIPOSA data, it’s essential to remember — this isn’t about competition. There is no one-size-fits-all “best” treatment for EGFR NSCLC. It’s about patients and families. Clinicians need the right tools to match the right treatment with the right patient.
That is why I implore companies to invest in research that helps us understand who benefits most from each approach, through biomarkers, disease characteristics, AND A REAL understanding of QOL from PROs (Safety/AE grades are endpoints for trials, not tolerability) – THAT is personalized medicine and how progress becomes meaningful. As the discussant Daniel Tan said, we need GPS maps to help guide patient treatment pathways. Options are hope, but only if we know how to navigate them.”
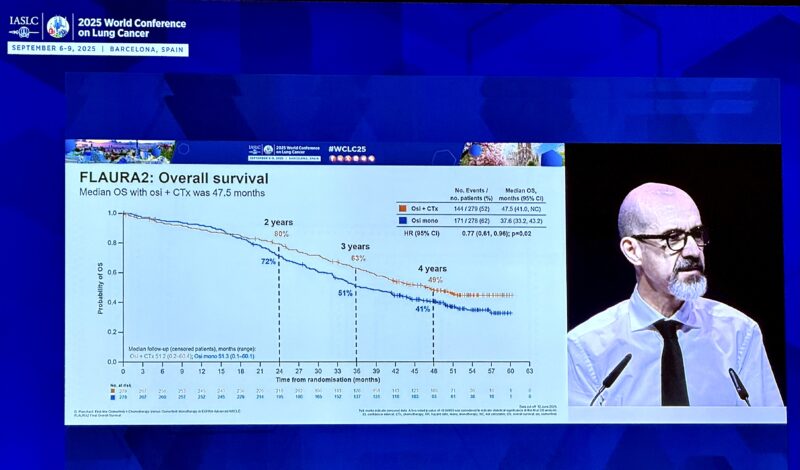
“Hot start to Presidential Symposium at WCLC25 from David Planchard with OS results from FLAURA2 – osi + chemo vs osi.
– mOS 47.5mo vs 37.6m (HR 0.77) 4 year.
– OS landmark 49% vs 41%.
– benefit observed across subgroups including mutation subtype and presence of CNS mets.”
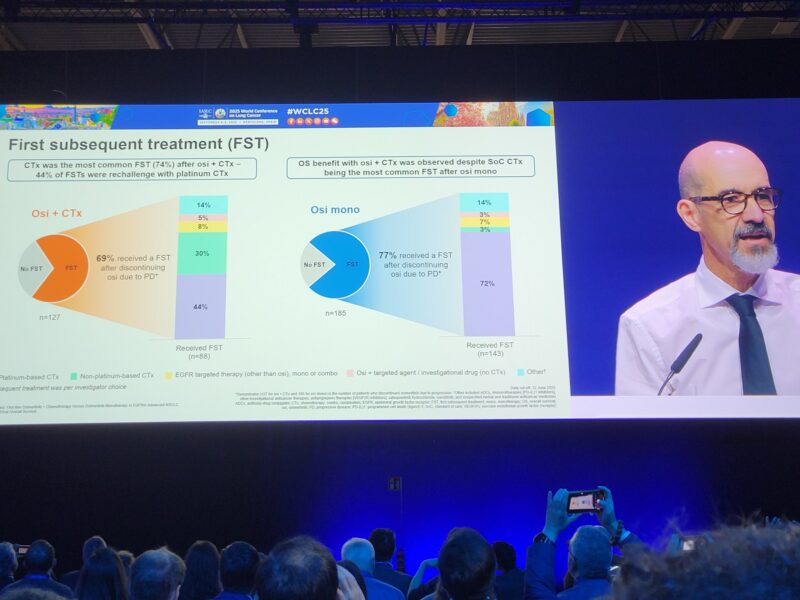
“FLAURA2 Overall survival presented at WCLC2025 showing an increase of survival of chemo +osi with 47.5 months HR 0.77 good news for our patients ”

“FLAURA2 Final OS at WCLC25
1L EGFRm NSCLC: Osi+Chemo vs Osi alone
mOS 47.5 vs 37.6 mo
HR 0.77 (95% CI 0.61–0.96; p=0.02)
3-yr OS: 63% vs 51%
Benefit consistent across subgroups
AEs: manageable, Osi discontinuation 12% vs 7%
Adds ~10 mo survival, but at cost of prolonged pemetrexed and real-world feasibility issues.
Takeaway: Osi+Chemo is new SOC, but Osi mono (mOS 37.6 mo) remains excellent for selected patients”
“Highly expected presentation WCLC 2025 Positive OS in FLAURA2 trial with “limited” exposure to chemo. Clear impact on pts outcome but also on pts journey…”
“FLAURA2 regimen (osimertinib + chemo) showed higher survival benefit over osimertinib alone in metastatic EGFR NSCLC. Where does this line with MARIPOSA with ami-laz?”
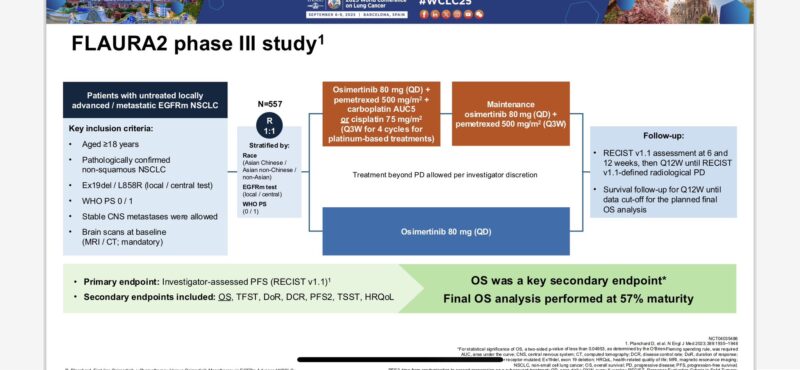
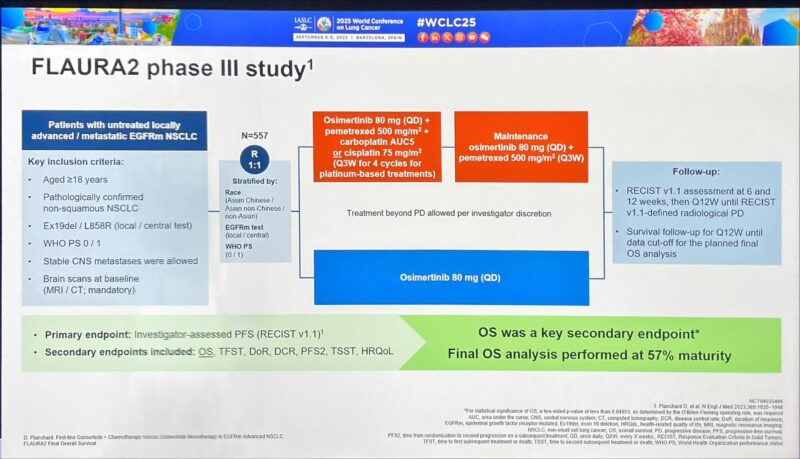
“FLAURA2 trial: Osimertinib + platinum-pemetrexed vs osimertinib
Study details
Phase III, randomized, open-label, global; 557 adults; ECOG 0–1
Stratified by
– mutation (Ex19del vs L858R),
– prior (neo)adjuvant chemo,
– CNS mets.
Primary: PFS (INV);
key secondary: OS;
other: ORR/DoR, QoL, TFST/TSST, safety.
Regimens
Combo: Osimertinib 80 mg QD + pemetrexed 500 mg/m² + cisplatin 75 mg/m² or carboplatin AUC5 q3w ×4 → maintenance osimertinib + pemetrexed q3w.
Control: Osimertinib 80 mg QD.
—
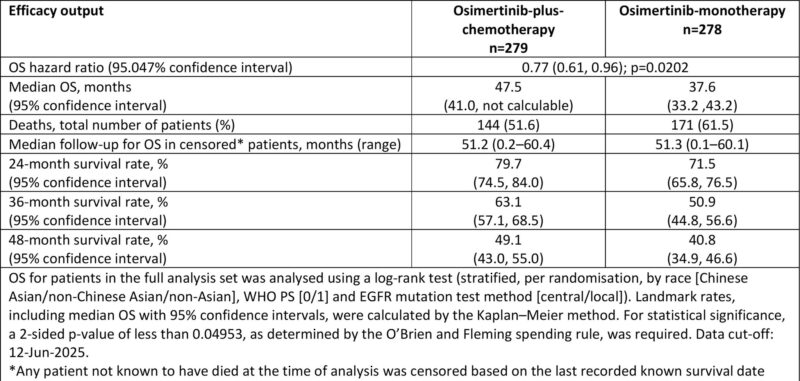
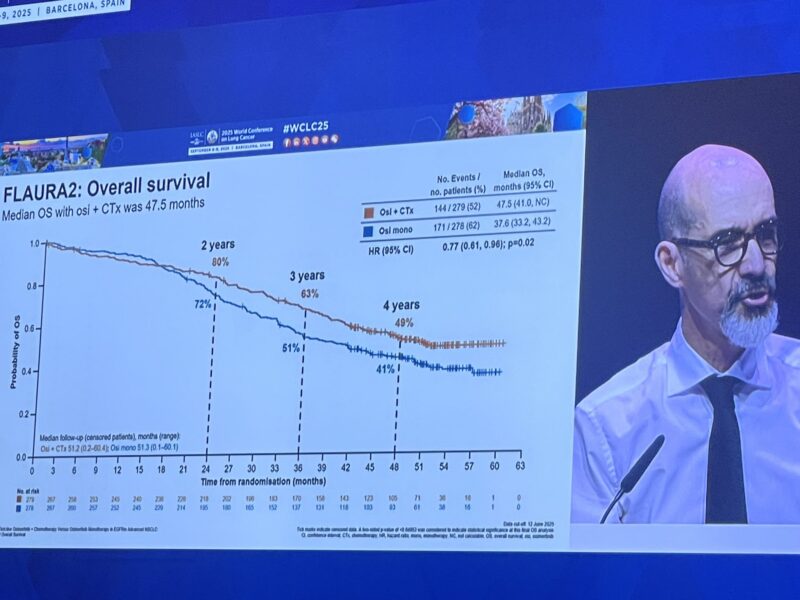
Final Overall Survival (WCLC 2025)
Median OS: 47.5 mo (combo) vs 37.6 mo (osi)
HR 0.77 , p value significant
OS rates:
24 mo 79.7% vs 71.5%;
36 mo 63.1% vs 50.9%;
48 mo 49.1% vs 40.8%.
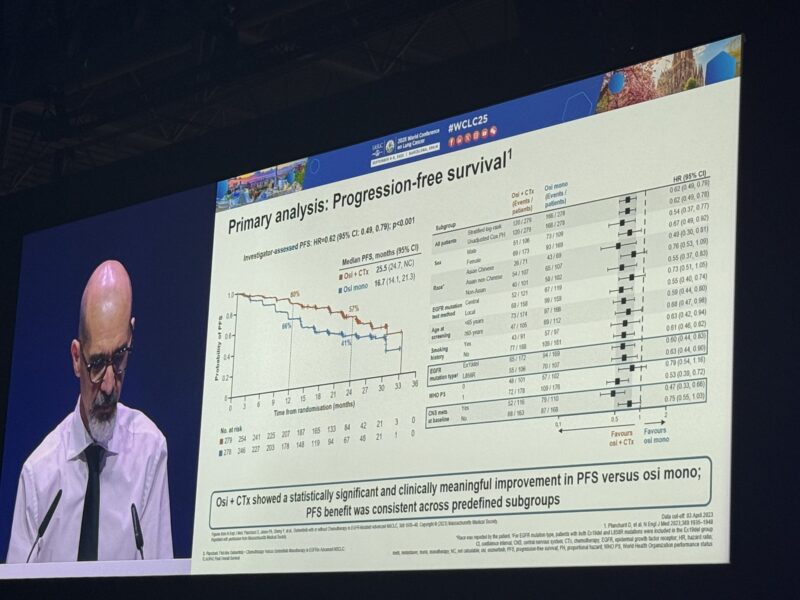
Progression-Free Survival (primary)
INV: 25.5 mo vs 16.7 mo;
HR 0.6 p<0.0001.
BICR : 29.4 mo vs 19.9 mo;
HR 0.62 , p=0.0002.
Responses
ORR: 83%
Median DoR: 24.0 mo vs 15.1–15.3 mo
CNS outcomes
CNS progression/death HR ≈0.58 ;
lower cumulative CNS-event risk with combo;
high CNS ORR and CR rates in evaluable set.
Safety (longer follow-up)
Grade ≥3 AEs: 70% (combo) vs 34% (osi);
discontinuations: 12% vs 7%;
no new safety signals;
toxicity profile as expected for pem-platinum + osimertinib.
—
Regulatory & “standard of care” context
FDA (Feb 16, 2024): 1L osimertinib + platinum-pemetrexed approved for EGFR Ex19del/L858R la/mNSCLC.
EU/EMA (July 2024): EU approval for the combo in 1L EGFRm advanced NSCLC.
Global adoption: Approved in >80 countries (incl. US, EU, China, Japan); WCLC-2025 final OS reinforces role.
—
How to use it (clinic take)
Choose combo when:
high disease burden,
baseline CNS involvement,
need for max depth/duration upfront; patient fit for chemo
Monotherapy reasonable when: chemo-ineligible/frail or strong preference to defer chemo.”
“FLAURA-2 meets the expectations. Final OS analyis: 10 months gain over osi alone, HR 0.77, total of 47.5 months Osi + Chemo. New landmark for EGFRmut lung cancer. Chemo rechallenge will become an option. Congrats @dplanchard
for a fantastic presentation.”
“Highly expected presentation WCLC2025 Positive OS in FLAURA2 trial with “limited” exposure to chemo. Clear impact on pts outcome but also on pts journey…”

“WCLC25 Presidential
FLAURA2 OS in 1L EGFR+ NSCLC:
– mOS 47.5m v 37.6m (HR 0.77 p=0.02)
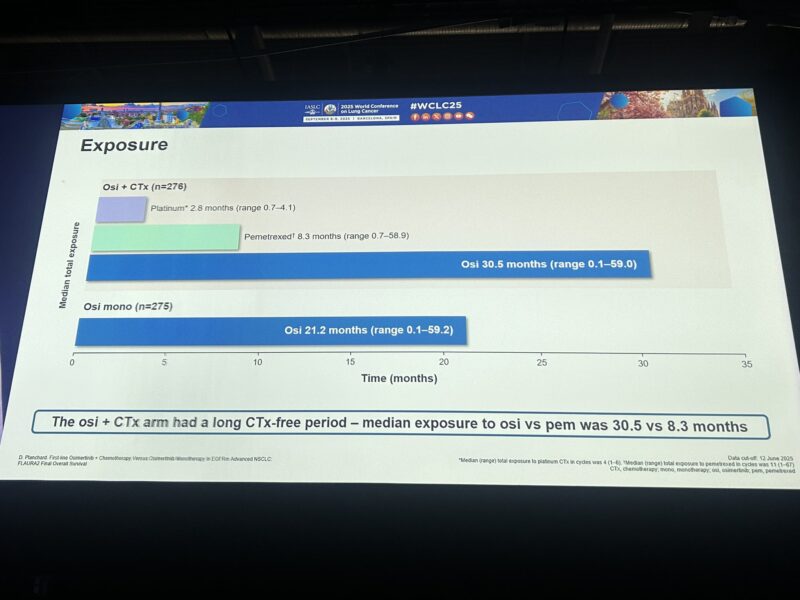
– duration of osi 30.5m on osi+chemo v 21.2m osi- combo delays acq resistance?
– 72% got 2L chemo- does this add ~9m?
– all subgrps benefit (?BM, ?co-mtns)
Congrats David Planchard.”

“Results from FLAURA2 presented at IASLC WCLC25. PFS previously presented (primary end point), superior with combination c/w osi alone. OS presented today, with a mOS 47.5mo Safety as previously reported. Fantastic presentation David Planchard!”
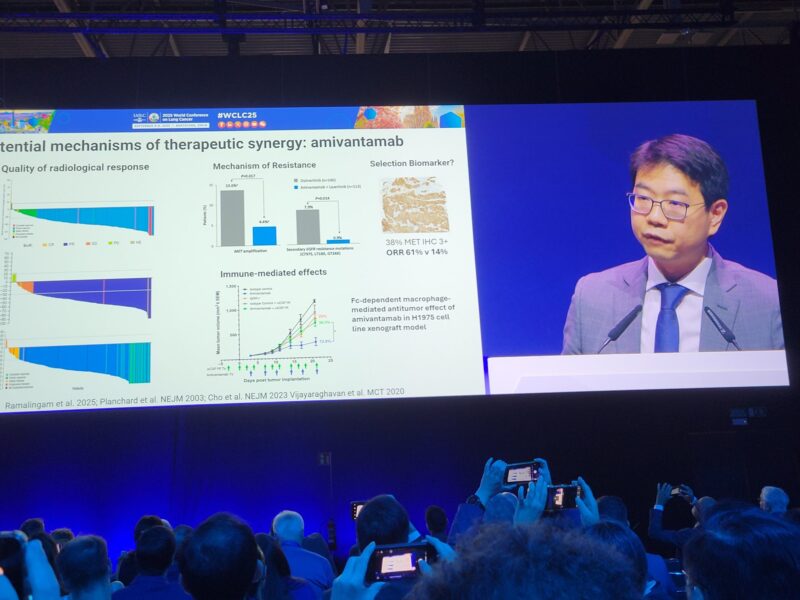
“Dr. Daniel Tan with an insightful discussion placing FLAURA2 in the context of the current and evolving landscape of EGFRm NSCLC.”
“FLAURA2 OS results Osimertinib + Chemotherapy in 1L EGFR mut NSCLC
Chemo + osimertinib median OS 47.5 mo vs 37.6 mo in osimertinib monotherapy Median exposure to pemetrexed only 8.3 mo in combination arm.
Many questions… Is the main effect from the 4 initial cycles of platinum-doublet? Can we use ctDNA to guide chemotherapy length? Important to study many of these questions in adaptive trials like Julia Rotow
and others ”
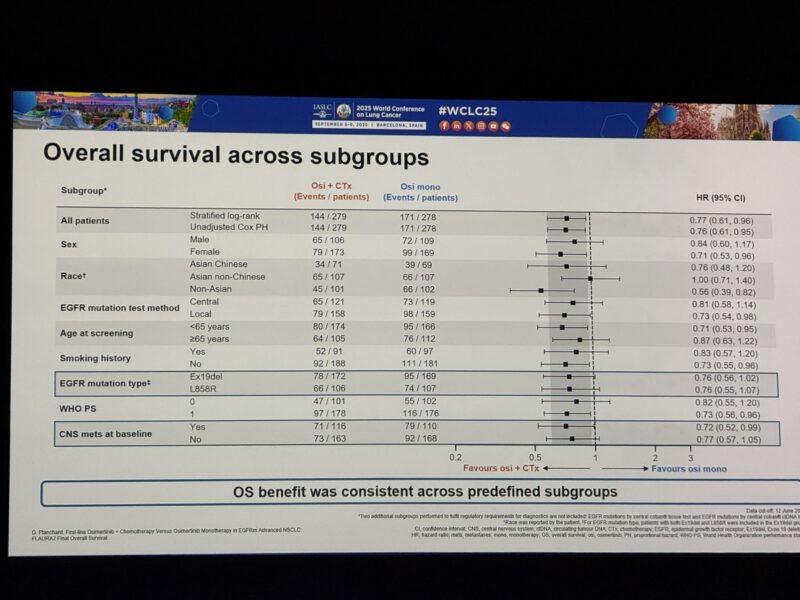
“Exciting final overall survival analysis of FLAURA2: osi + PT/pem vs osi alone for #EGFRm NSCLC WCLC25
OS with osi/CTx (median 47.5 mo) vs osi (37.6 mo) Benefit despite median exposure to pem 8.3 mo & most pts in control arm receiving subsequent PT-based chemo.”
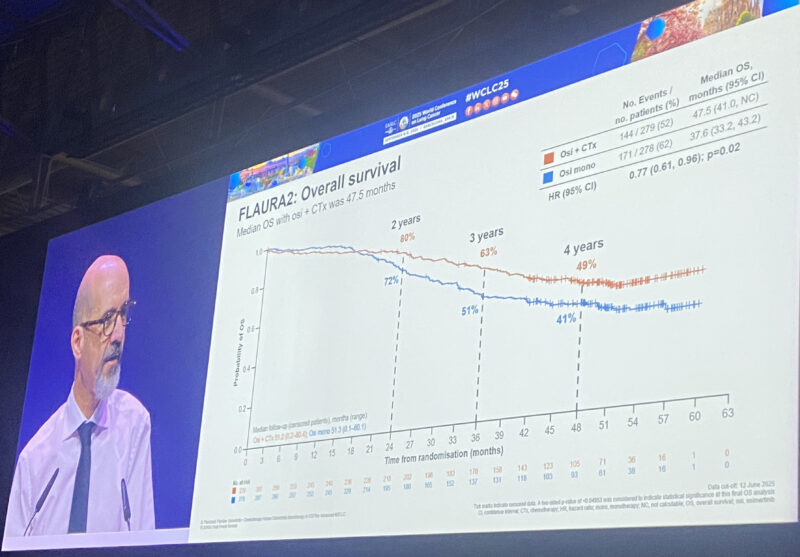
“Eventually we have the OS data from FLAURA2 presented by David Planchard at WCLC 25! Reassuring to see, that postPD treatment in the exp arm among progressors went 46% 69% ->platinum rechallenge is a feasible option, challenging the postPD lack of choices!”
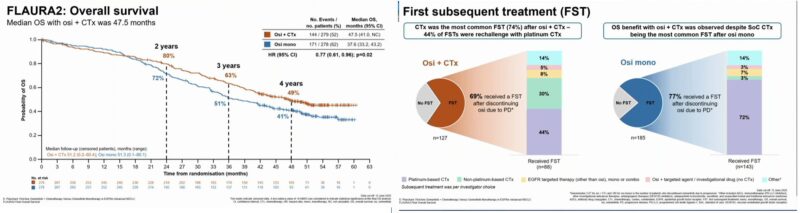
“FLAURA2 OS Results: In EGFRm advanced NSCLC, osimertinib + chemotherapy improved OS vs osimertinib alone (47.5 vs 37.6 mo; HR 0.77). Reinforces combinations as preferred first-line options.”
“Long-awaited OS results from #FLAURA2 (1L chemo + osimertinib vs osi): -mOS: 47.5 vs 37.6 mo (HR 0.77) -Benefit consistent across subgroups -Longer osimertinib exposure with combo Strong evidence supporting osi+chemo for newly diagnosed EGFR+ NSCLC.”

“David Planchard has just presented the OS data from the FLURA2. 12% difference at 3 years. Definitely a new standard of care. Waiting for the discussant Daniel Tan to guide us. ”

“Why are we seeing this degree of benefit in OS with the combination FLAURA2 regimen, as compared to single agent osimertinib?
Three proposed reasons:
1. Tumor responses
2. Impact on control
3. Subsequent therapy/crossover.”
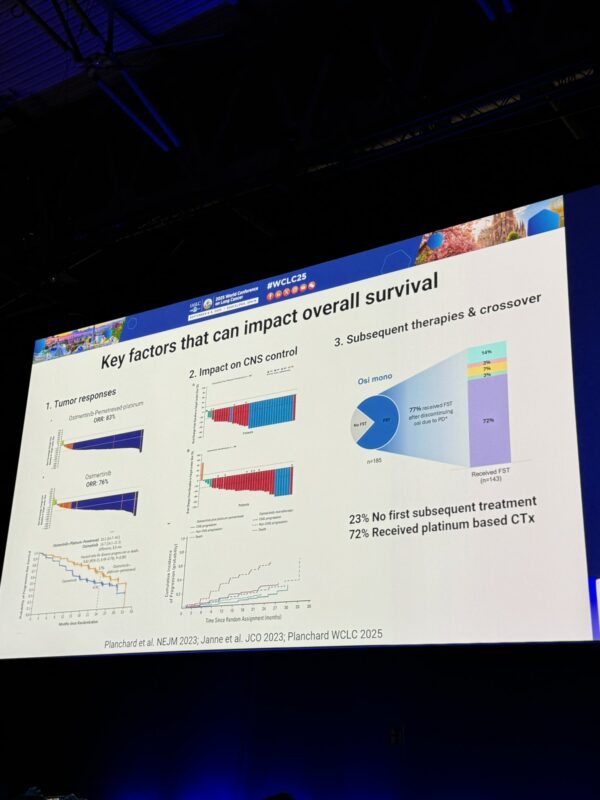
“WCLC25 Presidential symposium
Dr. Planchard presenting FLAURA2 OS data, HR =0.77, 12% absolute benefit at 3 yrs, with 72% of patients in Osi arm getting subsequent platinum chemo longer period of osi treatment duration in combo arm Median time on chemo ~8 months”
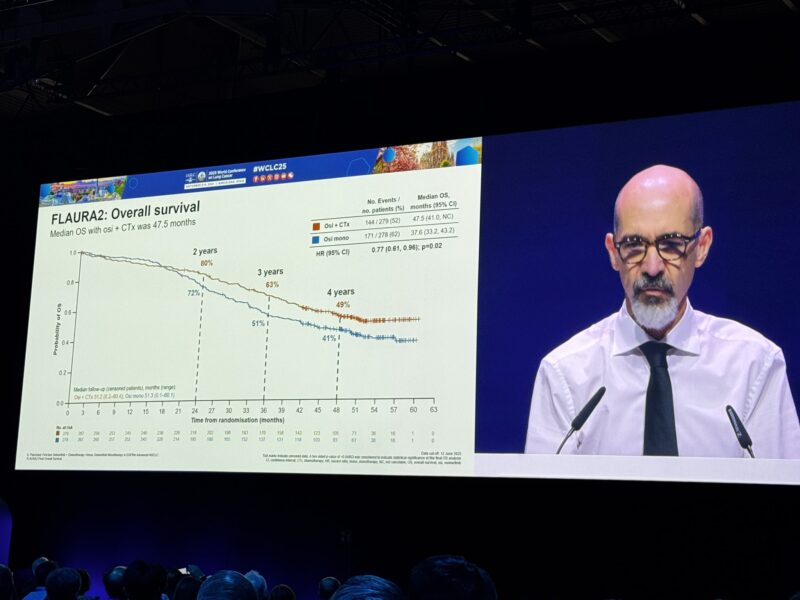
“WCLC25 | Final FLAURA2 results
Osi + chemo reached median OS 47.5 mo vs 36.8 mo with osi alone (HR 0.77; p=0.02).
At 4 yrs: 49% alive with combo vs 41% with osi mono.
Median osi exposure: 30.5 mo with combo vs 21.2 mo with osi mono.
Longer treatment exposure and OS benefit with manageable safety.”
“1L EGFRm NSCLC Data at Day-2 WCLC 2025:
Final OS data (HR 0.77) for FLAURA2 and promising PFS data (HR:0.55) for ACROSS2 trials presented today at WCLC2025. (EGFR-TKI in combo with chemo in 1L EGFRm NSCLC)
A side by side look with chemo-free MARIPOSA trial data.
Analysis
FLAURA2: Definitive → osimertinib + chemo is now the global SoC in 1L EGFRm NSCLC (broad population).
ACROSS2: Focused on difficult-to-treat subgroup (EGFRm + tumor suppressor mutations). Early but positive PFS signals; OS awaited.
MARIPOSA: Introduces novel EGFR/MET bispecific antibody strategy (amivantamab+lazertinib). Positioned as a non-chemo alternative to osimertinib.
Together:
Chemo backbone (FLAURA2) vs
Precision subset chemo (ACROSS2) vs
Biologic innovation (MARIPOSA)
This creates 3 complementary treatment strategies in the 1L EGFRm NSCLC landscape.
For in-depth trial related outcomes, explore.”


