
VERIFY Trial: Phase 3 Results of Rusfertide in Polycythemia Vera
The Phase 3 VERIFY trial (NCT05210790) evaluated the safety and efficacy of rusfertide in patients with PV who required regular phlebotomies despite standard therapy. The study was designed to confirm earlier Phase 2 data showing reductions in phlebotomy frequency, hematocrit stability, and symptom relief.
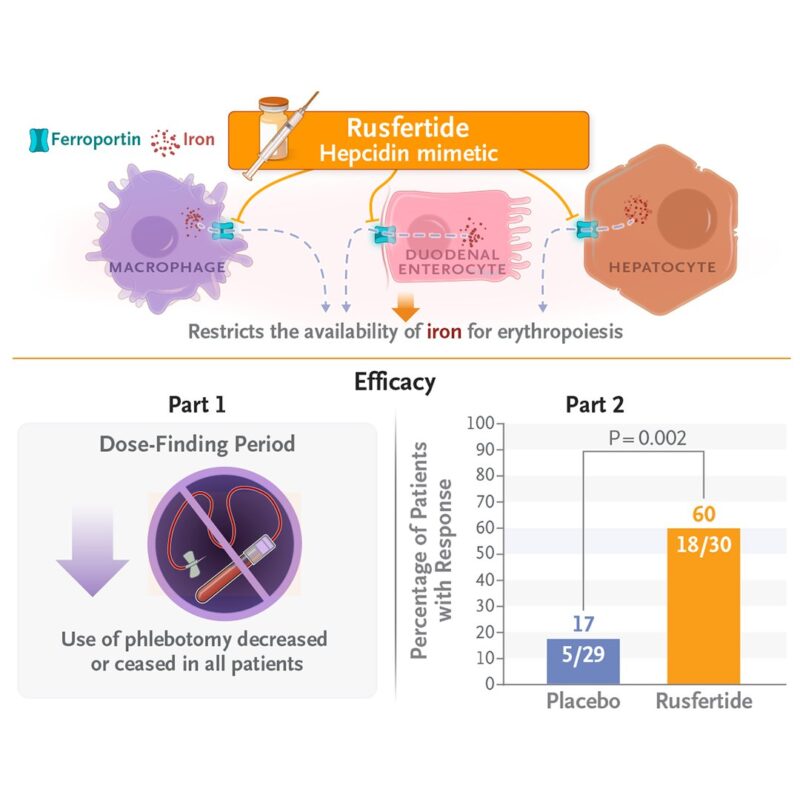
Trial Design
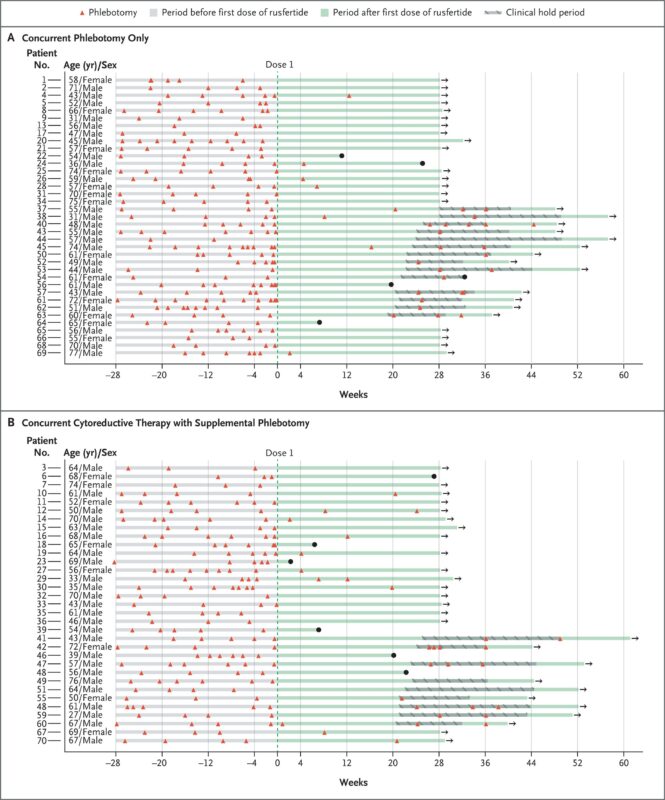
VERIFY was a randomized, double-blind, placebo-controlled Phase 3 trial enrolling 293 adult patients with polycythemia vera who were phlebotomy-dependent at baseline. All participants had undergone ≥3 phlebotomies in the prior 6 months or ≥6 in the prior year and were randomized to receive:
- Rusfertide (subcutaneous, once weekly), or
- Placebo, in addition to background standard therapy (e.g., aspirin, cytoreductive drugs)
The trial consisted of a 32-week blinded phase, followed by an open-label extension. The primary endpoint was clinical response—defined as maintenance of hematocrit <45% without phlebotomy eligibility between weeks 20 and 32.
Efficacy Results
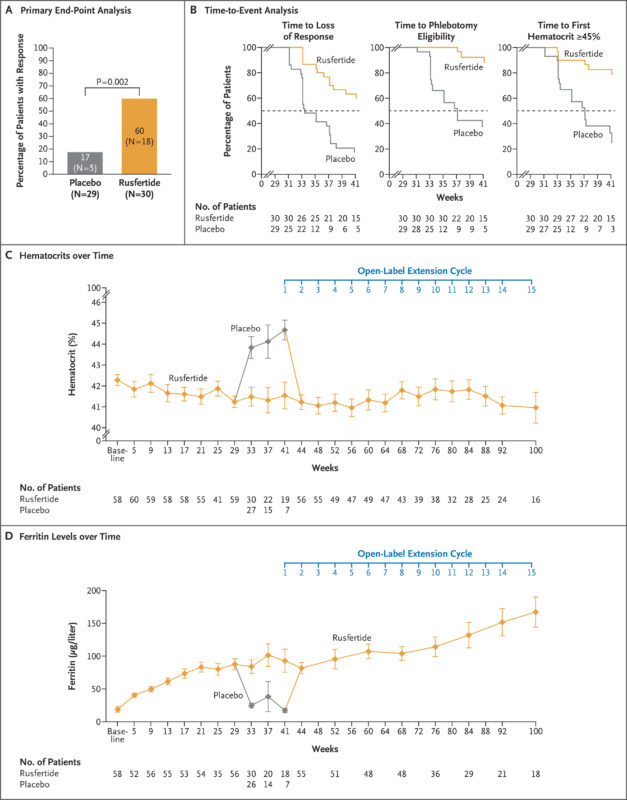
The VERIFY trial met its primary endpoint, demonstrating statistically and clinically significant benefit with rusfertide:
- 77% of patients in the rusfertide arm achieved a clinical response versus 33% in the placebo group (p < 0.0001).
- Rusfertide also led to a significant reduction in the number of phlebotomies, with a mean of 0.5 phlebotomies per patient in the treatment group compared to 1.8 in the placebo arm during the blinded phase (p < 0.0001).
- The drug maintained stable hematocrit control, reducing the need for reactive interventions.
Symptom and Quality of Life Outcomes
In addition to hematologic control, rusfertide was associated with meaningful improvement in patient-reported outcomes:
- Fatigue scores, assessed via the PROMIS Fatigue Short Form-8a, improved significantly in the rusfertide group.
- Symptom burden, measured using the Myeloproliferative Neoplasm Symptom Assessment Form (MFSAF) Total Symptom Score, also improved more in patients receiving rusfertide compared to placebo.
Safety Profile
Rusfertide was well tolerated, with a safety profile consistent with earlier studies. The most common adverse events were grade 1–2 injection site reactions, which were generally transient and manageable. Importantly, no new safety signals were observed, and there was no evidence of increased cancer risk or serious systemic toxicity during the trial.
Discussion
The Phase 3 VERIFY trial marks a pivotal development in the management of polycythemia vera (PV), particularly for patients who remain phlebotomy-dependent despite existing standard therapies. Rusfertide, a novel hepcidin mimetic, demonstrated clear clinical benefit in controlling hematocrit levels, reducing phlebotomy burden, and improving patient-reported outcomes. These results validate the therapeutic premise of targeting iron metabolism—a core physiological driver of erythrocytosis in PV.
The significant response rate (77% vs. 33% in placebo) and reduction in phlebotomy frequency underscore rusfertide’s potential to offer disease-modifying effects without the myelosuppressive risks associated with cytoreductive agents like hydroxyurea. Importantly, improvements in fatigue and total symptom scores further elevate its value, as symptom burden in PV has been historically under-addressed in clinical trials.
A major strength of the trial is its rigorous design—placebo-controlled and adequately powered to evaluate both efficacy and tolerability. The safety profile was favorable, with only low-grade injection site reactions being commonly reported, and no signals of serious adverse events or increased malignancy risk. These characteristics make rusfertide particularly appealing for patients who require long-term management and wish to avoid the risks associated with cytotoxic therapy.
However, certain limitations warrant consideration. The trial enrolled patients with established phlebotomy-dependence, and while this represents a clinically relevant group, the applicability of rusfertide in earlier disease stages or in combination with cytoreductive agents remains to be clarified. Additionally, long-term outcomes such as thrombotic event rates, leukemic transformation, and durability of symptom control will require extended follow-up through the open-label extension.
VERIFY’s findings also raise new questions about the potential repositioning of therapeutic goals in PV—from reactive phlebotomy toward proactive hematocrit stabilization with mechanistically targeted agents. As personalized treatment strategies continue to evolve, integrating biomarkers such as serum ferritin, hepcidin levels, or iron saturation could further refine patient selection and optimize treatment timing.
What is Polycythemia Vera
Polycythemia vera (PV) is a chronic myeloproliferative neoplasm characterized by excessive red blood cell production, often resulting in elevated hematocrit, thrombotic risk, and significant symptom burden. The current mainstay of treatment includes phlebotomy, cytoreductive agents (e.g., hydroxyurea), and low-dose aspirin. However, a substantial proportion of patients remain phlebotomy-dependent, with poorly controlled hematocrit and persistent symptoms. Rusfertide (PTG-300), a novel hepcidin mimetic, represents a targeted non-cytotoxic approach aimed at restoring iron homeostasis and controlling erythrocytosis.

Read More About Polycythemia Vera on Oncodaily
In summary, rusfertide represents an innovative and effective non-cytotoxic option for patients with polycythemia vera, and the VERIFY trial sets a new benchmark for evidence-based management of hematocrit and symptom burden. Its successful integration into practice will likely depend on long-term safety surveillance, regulatory approval, and incorporation into consensus guidelines.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
