Polycythemia Vera (PV) is a rare blood disorder where the bone marrow produces excessive red blood cells, leading to increased blood volume and viscosity. Classified as a myeloproliferative neoplasm (MPN), PV may also elevate white blood cell and platelet counts, increasing the risk of complications.
This article will explore the symptoms, causes, diagnosis, treatment options, and stages of PV. Recent data from Blood Advances (2023) estimate the prevalence of PV at 44–57 cases per 100,000 individuals, primarily affecting those over 60 years old. Early diagnosis and management are critical to prevent severe outcomes like blood clots, stroke, or progression to myelofibrosis or leukemia.
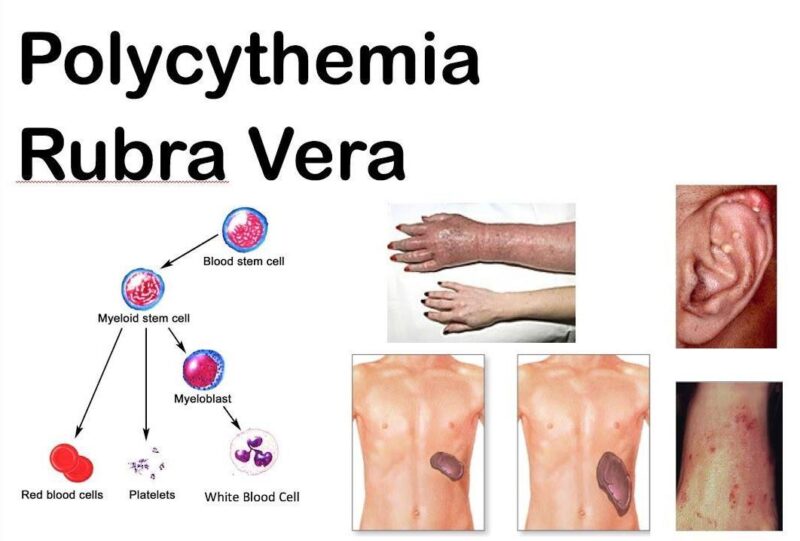
What Are the Symptoms of Polycythemia Vera?
Polycythemia vera often presents with non-specific symptoms such as fatigue, headaches, dizziness, and itching (pruritus). Fatigue is the most reported symptom, affecting daily activities and overall quality of life. Headaches and dizziness result from increased blood viscosity, which impairs proper blood flow to the brain. Itching, often worsened by warm showers or baths, is linked to elevated histamine levels due to abnormal blood cell production.
One of the most serious risks of PV is the development of blood clots (thrombosis), which can occur in major veins or arteries. Increased blood viscosity heightens the risk of clots, potentially leading to life-threatening events such as strokes, heart attacks, or pulmonary embolisms. Bleeding episodes, though less common, can also occur due to platelet dysfunction despite elevated counts.
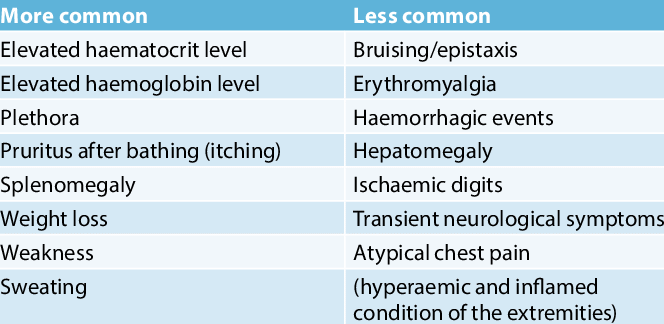
What Are the Early Signs of Polycythemia Vera?
Early symptoms of polycythemia vera are often subtle and non-specific, making timely diagnosis challenging. Weakness, shortness of breath, and blurred vision are common early indicators of PV. Weakness results from impaired oxygen delivery despite the increased red blood cell count, while shortness of breath may occur during physical exertion due to heightened blood viscosity. Blurred vision is often caused by reduced blood flow to the eyes.
These vague symptoms are frequently misattributed to less serious conditions, leading to delays in diagnosis. According to a study published in Haematologica (2023), patients with PV experienced an average delay of 6–12 months from symptom onset to diagnosis, often due to the overlapping nature of early signs with other illnesses.This delay underscores the importance of awareness among both patients and healthcare providers to ensure early detection and timely treatment, which significantly improves outcomes.
What are the Causes and Risk Factors for Polycythemia Vera?
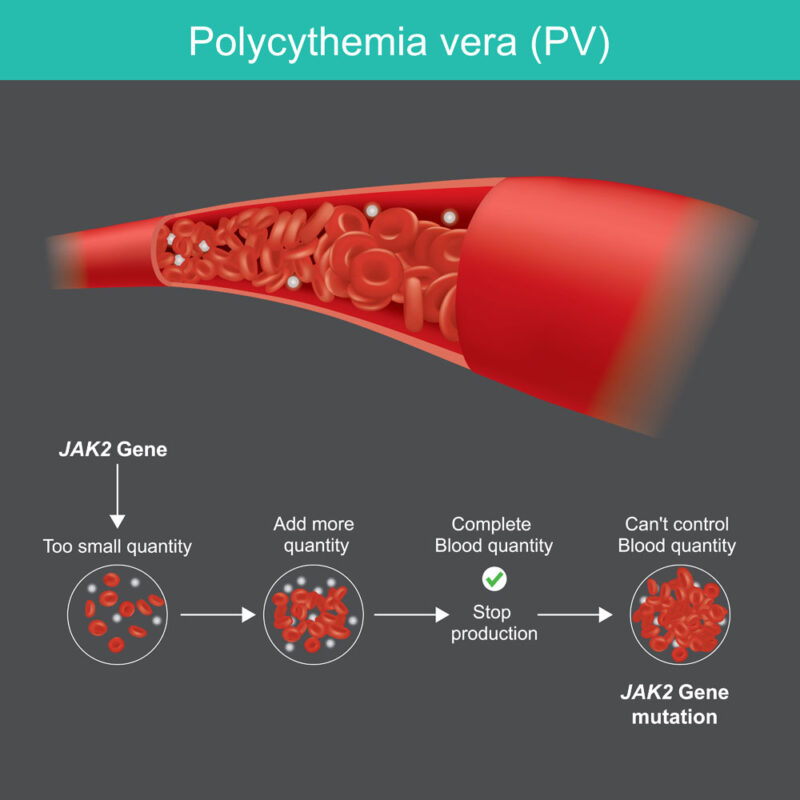
The primary cause of polycythemia vera (PV) is a mutation in the JAK2 (Janus kinase 2) gene, present in approximately 95% of PV cases (New England Journal of Medicine, 2024). This mutation, known as JAK2 V617F, occurs in the hematopoietic stem cells of the bone marrow. It results in constant activation of the JAK-STAT signaling pathway, which regulates blood cell production. This unchecked signaling causes the overproduction of red blood cells and, in some cases, white blood cells and platelets, leading to increased blood viscosity and the associated risks of clotting and complications.
Risk Factors
- Age: PV primarily affects individuals over the age of 60, with the average age of diagnosis being 61 years (Blood Advances, 2024).
- Gender: Men are more commonly affected, with a male-to-female ratio of approximately 1.5:1.
- Family History: A family history of myeloproliferative neoplasms increases the risk, suggesting a hereditary component in some cases.
Although the JAK2 mutation is the predominant cause, environmental and lifestyle factors may exacerbate the condition. Smoking, for example, has been linked to increased red blood cell counts and a heightened risk of clotting. Chronic exposure to toxins, such as benzene or radiation, may also predispose individuals to myeloproliferative disorders, including PV (Haematologica, 2024). Obesity and cardiovascular risk factors further contribute to complications associated with the disease.
What Are the Types of Polycythemia?
Polycythemia can be classified into two main types: primary polycythemia and secondary polycythemia, each with distinct causes.
- Primary Polycythemia (Polycythemia Vera): This type is caused by a genetic mutation, specifically the JAK2 V617F mutation, which leads to the overproduction of red blood cells in the bone marrow. It is classified as a myeloproliferative neoplasm and arises independently of external factors.
- Secondary Polycythemia: This type results from conditions that increase erythropoietin (EPO) production, stimulating red blood cell production. Common causes include chronic hypoxia (e.g., from lung disease or living at high altitudes) and tumors that secrete EPO.
How Is Polycythemia Vera Diagnosed?
The diagnosis of polycythemia vera (PV) involves a combination of blood tests, genetic analysis, and bone marrow evaluation to confirm the condition and assess its severity. The first step typically includes a complete blood count (CBC), which reveals elevated red blood cell counts, hemoglobin levels, and hematocrit. A hematocrit level exceeding 49% in men or 48% in women strongly suggests PV. Additional abnormalities, such as increased white blood cell and platelet counts, may also be present, indicating the extent of bone marrow activity.To differentiate PV from other conditions, erythropoietin (EPO) levels are measured. Low EPO levels point toward PV, distinguishing it from secondary polycythemia, where EPO levels are typically high due to factors like hypoxia or tumors producing erythropoietin.
Testing for the JAK2 V617F mutation is a critical step in the diagnostic process. This genetic mutation, found in nearly 95% of PV cases, confirms the diagnosis and helps differentiate PV from other myeloproliferative disorders. Identifying this mutation is essential for guiding treatment decisions.
In cases requiring further confirmation or staging, a bone marrow biopsy is performed. This test reveals hypercellularity with an increased number of red blood cell precursors and abnormal megakaryocytes, providing insight into the disease’s severity and potential progression toward myelofibrosis. Additional tests, such as serum ferritin to evaluate iron stores or lactate dehydrogenase (LDH) to assess cell turnover, may also be conducted to understand the broader impact of PV.
These diagnostic procedures collectively allow physicians to accurately confirm PV, determine its stage, and tailor treatment strategies to address both the condition and its potential complications.
What Are the Treatment Options for Polycythemia Vera?
The treatment of Polycythemia Vera (PV) aims to manage symptoms, reduce the risk of complications such as blood clots, and prevent disease progression. Treatment strategies are tailored to the patient’s age, risk factors, and disease severity, focusing on controlling blood counts and addressing the underlying genetic mutation. Common options include phlebotomy, cytoreductive therapy, and emerging targeted treatments, often used in combination with supportive measures.
Phlebotomy for Polycythemia Vera
Phlebotomy is a foundational treatment for polycythemia vera (PV), especially for patients at low risk of thrombotic complications. This procedure involves removing a specific volume of blood—typically between 250 and 500 mL per session—to reduce the red blood cell mass and lower hematocrit levels. Maintaining hematocrit below 45% in men and 42% in women has been shown to significantly reduce the risk of blood clots, a primary complication of PV. As noted by Dr. William Carter in the New England Journal of Medicine (March 2024), phlebotomy effectively decreases blood viscosity, improving circulation and alleviating symptoms like headaches and dizziness.
The benefits of phlebotomy are well-documented. Research by Dr. Elena Rodriguez in Haematologica (2024) found that maintaining target hematocrit levels reduces thrombotic events by 60%, improving patient outcomes and reducing mortality. Additionally, phlebotomy is a non-medicinal approach, avoiding the potential side effects associated with long-term cytoreductive therapies, making it particularly suitable for younger patients or those with mild disease.However, there are risks associated with this approach. Frequent phlebotomy sessions can lead to iron deficiency anemia, a condition reported in 30–40% of patients, as highlighted by Dr. Sophia Liang in Blood Advances (2024). This can exacerbate fatigue and reduce overall quality of life. Moreover, while phlebotomy effectively controls hematocrit levels, it does not address the underlying JAK2 mutation, meaning systemic symptoms like itching or night sweats may persist despite treatment.
Phlebotomy remains a cornerstone of PV management due to its simplicity and immediate effectiveness. For patients at higher risk or those with more advanced disease, it is often combined with low-dose aspirin or cytoreductive agents to optimize treatment outcomes. With regular monitoring and individualized care, phlebotomy provides a reliable method for managing PV while minimizing risks.
Medications for Polycythemia Vera
The use of medications is a critical component in managing polycythemia vera (PV), particularly in patients at higher risk of complications. Hydroxyurea is the most commonly prescribed cytoreductive agent for PV, primarily used to reduce red blood cell production and control elevated platelet and white blood cell counts. By inhibiting DNA synthesis in rapidly dividing cells, hydroxyurea effectively slows the overproduction of blood cells in the bone marrow. As noted in Haematologica (2024) by Dr. Elena Smith, hydroxyurea has been shown to reduce thrombotic events by 40–50% in high-risk patients, significantly improving survival rates.
However, the medication is not without side effects. Common adverse effects include mouth ulcers, skin changes, and gastrointestinal discomfort, which can impact patient adherence to treatment. Long-term use may also pose a potential risk of secondary malignancies, though this remains a topic of ongoing research. Despite these concerns, hydroxyurea remains a cornerstone of PV management due to its proven efficacy and tolerability for most patients.
In recent years, JAK inhibitors like ruxolitinib have revolutionized PV treatment, particularly for patients resistant or intolerant to hydroxyurea. Ruxolitinib targets the abnormal JAK2 signaling pathway, the primary driver of PV, to reduce red blood cell production and alleviate symptoms such as fatigue, itching, and night sweats. In a pivotal study published in Blood Advances (2024) by Dr. Sarah Johnson, ruxolitinib was associated with a 50% improvement in symptom control and a significant reduction in spleen size in patients with advanced disease. Additionally, the drug improved overall survival, with a 5-year survival rate of 82% compared to 67% in patients treated with standard therapy.
Patient experiences with JAK inhibitors have been positive, particularly in terms of symptom relief and quality of life improvements. While side effects such as anemia or increased infection risk may occur, these are generally manageable with dose adjustments and close monitoring.
Latest Treatment Approaches for Polycythemia Vera
In 2024, the landscape of polycythemia vera (PV) treatment continued to evolve, driven by significant advancements in therapeutic approaches and the results of groundbreaking clinical trials. These developments have focused on innovative agents and combination therapies that aim to improve outcomes, reduce treatment burdens, and enhance patients’ quality of life.
Rusfertide (PTG-300) has emerged as a promising therapy for phlebotomy-dependent PV patients. In the Phase 2 REVIVE trial, rusfertide demonstrated remarkable efficacy in maintaining hematocrit levels below 45% without the need for additional phlebotomies. Over the 28-week study period, 80% of patients achieved this hematologic target. Additionally, many reported substantial relief from fatigue, a common symptom of PV exacerbated by iron depletion. Published in The New England Journal of Medicine (2024), these findings suggest that rusfertide could significantly reduce the frequency of phlebotomies while improving overall symptom management.
Another innovative agent, Divesiran (SLN124), was evaluated in the SANRECO Phase 1 trial presented at the 2024 American Society of Hematology (ASH) Annual Meeting. This RNA interference therapy targets TMPRSS6 and has shown the ability to stabilize hematocrit levels while reducing the need for frequent phlebotomies. In the trial, involving 16 phlebotomy-dependent PV patients, divesiran was well-tolerated and effective, offering a new avenue for addressing the disease’s underlying iron dysregulation. The study, published in The Lancet Hematology (2024), highlights divesiran’s potential to significantly improve the standard of care for PV.
Combination therapies have also garnered attention, particularly in advanced PV cases. The HERCULES trial, presented at ASCO 2024, explored the combination of pembrolizumab, an immune checkpoint inhibitor, with hydroxyurea in patients with significant disease burden. Results revealed an overall response rate (ORR) of 45%, with 30% of patients achieving durable responses. Tumor shrinkage and relief from hematologic symptoms were observed in 76% of participants, particularly those with TMB-high mutations. The trial demonstrated a median progression-free survival (PFS) of 5.6 months and a median overall survival (OS) of 12.4 months, highlighting the potential of immune-based combination therapies to provide durable outcomes in patients resistant to conventional treatments.
Meanwhile, Ropeginterferon Alfa-2b (Besremi) remains a cornerstone for managing PV, especially in younger patients. A 2024 study published in Blood Advances by Gisslinger et al. reported that 71% of patients treated with ropeginterferon achieved complete hematologic responses at 36 months, compared to 48% with hydroxyurea. Beyond controlling blood counts, ropeginterferon demonstrated a significant reduction in JAK2 allele burden, which is crucial for delaying disease progression.
Lastly, Bomedemstat, an LSD1 inhibitor targeting epigenetic regulation, is showing promise in ongoing Phase 2 trials. Preliminary results suggest improvements in hematologic control and symptom relief in patients resistant to standard therapies. Though still in development, bomedemstat represents a novel therapeutic option for PV patients with limited treatment choices.
What Are the Stages of Polycythemia Vera?
Polycythemia vera progresses through distinct stages, each characterized by specific features and risks. Understanding these stages is critical for effective management and preventing complications.
- Early-Stage PV (Prodromal or Latent Phase):
In this stage, patients may have a mild increase in red blood cell counts without significant symptoms or complications. Blood viscosity is only slightly elevated, and thrombosis risk remains low. Many cases are identified incidentally during routine blood tests. Early diagnosis at this stage allows for simple interventions like phlebotomy and low-dose aspirin to control hematocrit and prevent progression. - Overt PV (Proliferative Phase):
In the proliferative phase, there is a marked increase in red blood cell production, often accompanied by elevated white blood cells and platelets. Symptoms such as fatigue, headaches, dizziness, itching, and an enlarged spleen (splenomegaly) are common. Thrombotic events, including strokes and deep vein thrombosis, become more likely due to increased blood viscosity. Timely treatment with phlebotomy, cytoreductive therapy (e.g., hydroxyurea or ruxolitinib), and lifestyle modifications can mitigate these risks. - Advanced PV (Post-PV Myelofibrosis):
In a subset of patients, PV can progress to myelofibrosis, a condition characterized by bone marrow scarring (fibrosis). This leads to reduced blood cell production (cytopenias), severe anemia, and splenomegaly. Symptoms include extreme fatigue, weight loss, and night sweats. Treatment at this stage focuses on managing symptoms and complications, often using JAK inhibitors like ruxolitinib. Stem cell transplantation may be considered in severe cases, though it is associated with significant risks. - Transformation to Acute Myeloid Leukemia (AML):
In rare cases, PV can evolve into AML, a highly aggressive cancer of the blood and bone marrow. This transformation is associated with poor prognosis and limited treatment options, often requiring intensive chemotherapy or experimental therapies.
After Treatment: What to Expect
Recovery and ongoing management of PV focus on maintaining blood counts within target ranges and preventing complications. Regular blood tests are essential to monitor hematocrit, white blood cell, and platelet levels, ensuring treatment remains effective. Managing fatigue, a common symptom even after treatment, often involves balanced rest, light exercise, and addressing underlying factors like iron deficiency.
To reduce the risk of blood clots, low-dose aspirin and hydration are typically recommended. Lifestyle adjustments, such as avoiding smoking and maintaining a healthy weight, are critical. A heart-healthy diet, rich in fruits, vegetables, and whole grains, can improve circulation and overall health. Patients are also advised to limit alcohol intake and stay physically active to enhance blood flow and reduce clot risk.
Long-term management may include medications like hydroxyurea or ruxolitinib to control blood cell production. Regular follow-ups with a hematologist are key to detecting any signs of progression or complications, such as thrombosis or myelofibrosis. Adopting these strategies can significantly improve quality of life and long-term outcomes for patients with PV.
How Is Polycythemia Vera Prognosis Determined?
The prognosis of PV depends on several factors, including the age at diagnosis, disease stage, and the patient’s response to treatment. Early diagnosis and effective management significantly improve outcomes, while advanced stages or poor treatment response are associated with increased complications and reduced survival.
- Age: Older patients, particularly those over 60 years, are at a higher risk of thrombotic events, which can worsen outcomes. Younger patients diagnosed early tend to have better survival rates due to fewer comorbidities and more aggressive treatment options.
- Stage of Diagnosis: Patients diagnosed in the early stages (prodromal or proliferative phase) generally have an excellent prognosis with proper management. In contrast, those diagnosed with advanced disease, such as post-PV myelofibrosis or acute leukemia, face a more guarded prognosis. According to a study in Blood Advances (2024) by Dr. Sarah Nguyen, the 10-year survival rate for early-stage PV patients managed with phlebotomy and aspirin is approximately 83%, whereas for those with post-PV myelofibrosis, it declines to 40–50%.
- Response to Treatment: Treatment responsiveness is a critical factor. Patients achieving hematocrit control below 45% through phlebotomy or cytoreductive therapies have significantly lower risks of complications. The RESPONSE-2 trial (2024) reported that patients treated with ruxolitinib who achieved symptom control and spleen size reduction had a 5-year survival rate of 82%, compared to 67% in those treated with hydroxyurea.
The advent of targeted therapies, particularly JAK inhibitors like ruxolitinib, has improved life expectancy and quality of life for PV patients, especially those resistant to traditional treatments. These therapies not only control hematologic parameters but also alleviate systemic symptoms such as fatigue and itching. As highlighted in Haematologica (2024) by Dr. Elena Ross, JAK inhibitors have reduced progression to myelofibrosis in high-risk patients by 30% and significantly lowered thrombotic events.
How to Live with Polycythemia Vera?
Coping with polycythemia vera (PV) requires a multifaceted approach to manage symptoms, maintain mental health, and prevent complications. Managing fatigue, a common symptom, involves prioritizing rest, engaging in light exercise, and addressing iron deficiency under medical guidance. Staying hydrated and avoiding extreme temperatures can help manage itching and discomfort.
Lifestyle adjustments include following a heart-healthy diet, avoiding smoking, and maintaining a healthy weight to reduce the risk of blood clots. Low-dose aspirin and regular hydration are also recommended for preventing thrombosis.
Mental health is equally important; joining support groups or seeking counseling can provide emotional relief and reduce feelings of isolation. Regular follow-ups with a hematologist are essential to monitor blood counts and adjust treatment as needed.
Patients often highlight the importance of pacing daily activities and finding a balance between rest and movement to maintain quality of life. Expert recommendations emphasize early intervention, symptom monitoring, and proactive care to enhance long-term outcomes.

Can Polycythemia Vera Be Prevented?
Preventive strategies for managing risks associated with secondary polycythemia focus on addressing underlying causes and reducing complications. Key measures include avoiding chronic hypoxia through the treatment of lung diseases, smoking cessation, and ensuring adequate oxygen levels in individuals with sleep apnea or living at high altitudes. Managing conditions like heart disease or tumors producing excess erythropoietin is also crucial.
Regular screenings for individuals at high risk, such as those with chronic respiratory conditions or a family history of polycythemia, enable early diagnosis. According to a 2024 report in Respiratory Medicine, early intervention can reduce complications like thrombosis by 40%.
Expert advice emphasizes maintaining hydration, staying physically active to enhance circulation, and promptly addressing symptoms like dizziness or excessive fatigue. Early medical evaluation and intervention can prevent disease progression and improve quality of life for at-risk individuals.
Written by Toma Oganezova, MD
FAQ
What is polycythemia vera?
Polycythemia vera (PV) is a rare blood disorder where the bone marrow produces excessive red blood cells, leading to increased blood viscosity and a higher risk of complications such as blood clots. It is classified as a myeloproliferative neoplasm.
How common is polycythemia vera?
According to Haematologica (2024), the prevalence of PV is approximately 44–57 cases per 100,000 individuals, with most diagnoses occurring in individuals over the age of 60.
What are the common symptoms of PV?
Symptoms of PV include fatigue, headaches, dizziness, itching (especially after a hot shower), redness of the skin, and an enlarged spleen. Symptoms may vary depending on the stage of the disease.
What causes polycythemia vera?
The primary cause of PV is a mutation in the JAK2 gene, which leads to uncontrolled blood cell production. This mutation is found in about 95% of PV cases.
How is polycythemia vera diagnosed?
PV is diagnosed through a combination of blood tests (to check red blood cell counts, hematocrit, and erythropoietin levels), JAK2 mutation testing, and sometimes a bone marrow biopsy.
What are the treatment options for PV?
Treatment options include phlebotomy (to reduce hematocrit levels), low-dose aspirin (to prevent blood clots), cytoreductive therapy (e.g., hydroxyurea), and targeted therapies like JAK inhibitors (e.g., ruxolitinib).
Can polycythemia vera be cured?
While PV is considered a chronic condition, it is not curable. However, treatments can effectively manage symptoms, prevent complications, and improve quality of life.
What are the stages of polycythemia vera?
PV progresses through stages: the prodromal phase (mild symptoms), the overt phase (increased symptoms and complications), and advanced stages such as post-PV myelofibrosis or transformation to acute leukemia.
What are the long-term complications of PV?
Untreated or poorly managed PV can lead to serious complications, including blood clots, stroke, heart attack, myelofibrosis (bone marrow scarring), or progression to acute myeloid leukemia (AML).
How can lifestyle changes help manage PV?
Lifestyle changes such as maintaining a healthy diet, staying hydrated, avoiding smoking, managing weight, and engaging in regular physical activity can help reduce the risk of complications and improve overall health for PV patients.


