Unresectable and metastatic esophageal squamous cell carcinoma (ESCC) remains a highly lethal malignancy, with poor long-term survival despite recent advances in first-line immunotherapy. Although combinations of PD-1 or PD-L1 inhibitors with platinum-based chemotherapy have become standard of care, clinical outcomes remain suboptimal, and there is no global consensus on the optimal frontline regimen.
Targeting multiple immune checkpoints simultaneously has emerged as a rational strategy to further enhance antitumor immunity. Preclinical models and early-phase clinical studies suggested that dual inhibition of TIGIT and PD-L1 could potentiate immune activation beyond PD-1/PD-L1 blockade alone by enhancing T-cell and natural killer (NK) cell–mediated antitumor immunity.
In this context, the phase III SKYSCRAPER-08 trial evaluated whether the addition of the anti-TIGIT antibody tiragolumab to atezolizumab plus platinum–taxane chemotherapy could improve outcomes in the first-line treatment of unresectable or metastatic ESCC. The study, titled “Tiragolumab plus atezolizumab and chemotherapy as first-line treatment for patients with unresectable oesophageal squamous cell carcinoma (SKYSCRAPER-08): a randomised, double-blind, placebo-controlled, phase 3 trial,” was published in January 2026 in The Lancet Oncology.
Authors: Chih-Hung Hsu, MD, Zhihao Lu, MD, Shegan Gao, MD, Junye Wang, MD, Jong-Mu Sun, MD, Tianshu Liu, MD, Qingxia Fan, MD, Jun Cai, BSc, Feijiao Ge, MD, Sijing Li, PhD, Li Zhang, PhD, Edward Cha, MD, Simon Allen, PhD, Lin Shen, MD.
What is Tiragolumab and how does it work?
Tiragolumab is an investigational immunotherapy and a humanized monoclonal antibody targeting TIGIT, an inhibitory immune checkpoint expressed on activated T cells and natural killer (NK) cells.
Physiologically, TIGIT limits immune activation by binding to its ligand CD155 on tumor cells and antigen-presenting cells, resulting in reduced T-cell proliferation, cytokine production, and NK-cell cytotoxicity. Tumors exploit this pathway to suppress antitumor immune responses.
Tiragolumab blocks TIGIT–CD155 interactions, thereby releasing inhibitory signaling and enhancing effector T-cell and NK-cell–mediated antitumor immunity. Because TIGIT signaling is complementary to the PD-1/PD-L1 axis, tiragolumab has been developed primarily in combination with PD-L1 inhibitors such as Atezolizumab to enhance antitumor immunity through dual checkpoint blockade.
Key scientific points
- Target: TIGIT immune checkpoint
- Mechanism: Inhibition of TIGIT-mediated immune suppression
- Rationale: Synergistic activation with PD-1/PD-L1 blockade
Clinical studies have shown early signs of activity in selected populations; however, later-phase trials have reported heterogeneous outcomes. Tiragolumab therefore remains under clinical investigation and is not yet established as standard therapy.
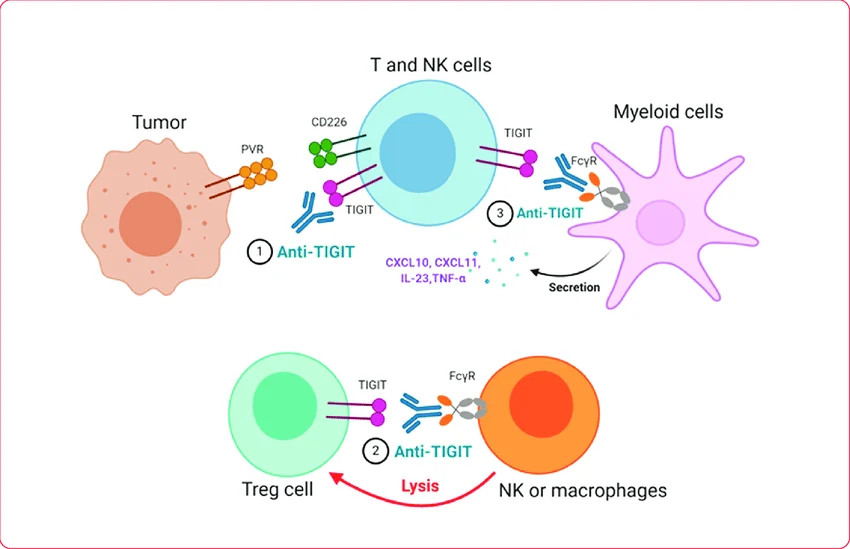
Source: Tiragolumab. Image republished from Yeo et al. (2021) under terms of Creative Commons Attribution License (CC BY).
Methods
SKYSCRAPER-08 was a randomized, double-blind, placebo-controlled phase III trial conducted across 67 centers in mainland China, South Korea, Thailand, Taiwan, and Hong Kong. Adult patients with treatment-naïve, unresectable locally advanced, unresectable recurrent, or metastatic ESCC and ECOG performance status 0–1 were eligible.
Patients were randomized 1:1 to receive either tiragolumab plus atezolizumab combined with cisplatin and paclitaxel, or placebo plus chemotherapy. Treatment consisted of six 21-day induction cycles followed by maintenance immunotherapy or placebo until disease progression or unacceptable toxicity.
Randomization was stratified by PD-L1 tumor area positivity score (<10% vs ≥10%), ECOG performance status (0 vs 1), and prior curative treatment (yes vs no). Tumor assessments were performed by an independent review facility using RECIST v1.1.
Endpoints
The dual primary endpoints were independent review facility-assessed progression-free survival (PFS) and overall survival (OS) in the intention-to-treat population.
Key secondary endpoints included objective response rate, duration of response, investigator-assessed PFS, and safety. Patient-reported outcomes and quality-of-life analyses were prespecified but are to be reported separately.
Results
Between October 2020 and November 2021, 461 patients were enrolled and randomized. Baseline characteristics were well balanced between treatment arms. The study population was entirely Asian, and nearly 90% of patients were male, reflecting the epidemiology of ESCC in East Asia.
At the primary PFS analysis, the addition of tiragolumab and atezolizumab to chemotherapy resulted in a statistically significant improvement in median PFS compared with chemotherapy alone. Median PFS was 6.2 months in the dual immunotherapy arm versus 5.4 months in the control arm (HR 0.56), corresponding to a 44% relative reduction in the risk of disease progression or death.
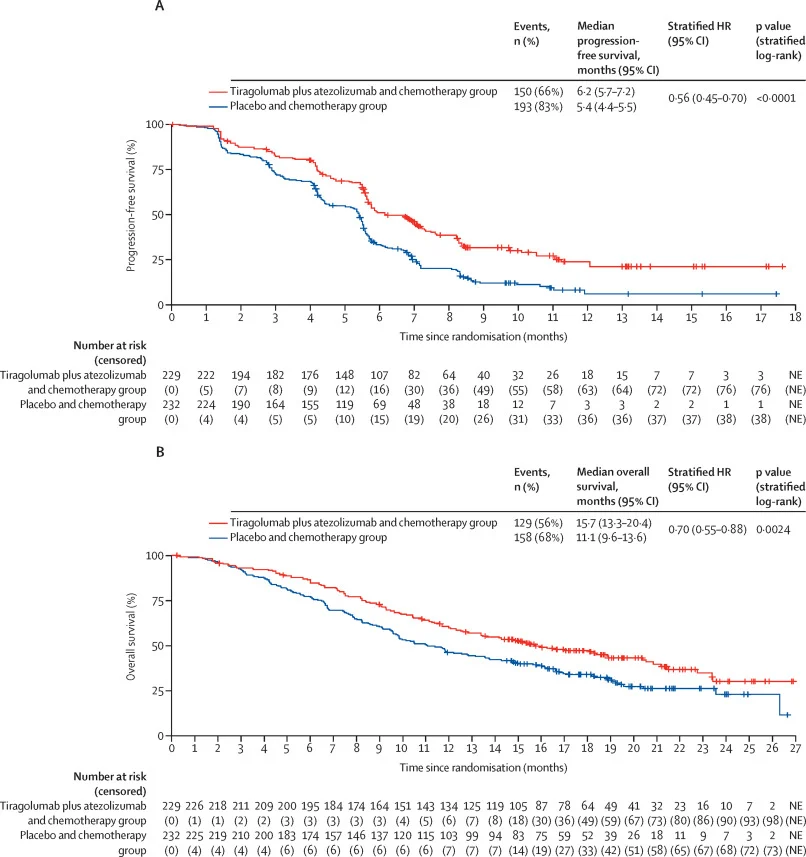
Overall survival was also significantly prolonged. Median OS reached 15.7 months with tiragolumab plus atezolizumab and chemotherapy, compared with 11.1 months in the placebo plus chemotherapy group. Survival benefits were generally consistent across prespecified subgroups, including patients with low or negative PD-L1 expression, although confidence intervals were wide and crossed unity in smaller subgroups, limiting definitive conclusions.
Objective response rates were higher with dual checkpoint inhibition, and responses were more durable than with chemotherapy alone. Investigator-assessed outcomes were concordant with independent review findings.
Safety
The safety profile of tiragolumab plus atezolizumab and chemotherapy was consistent with known toxicities of chemotherapy and immune checkpoint inhibition. Grade 3–4 adverse events were more frequent in the dual immunotherapy arm, largely reflecting longer treatment exposure.
Immune-mediated adverse events, including rash, hepatitis, and pneumonitis, occurred more often with tiragolumab plus atezolizumab but were generally manageable. The most common grade 3–4 adverse events were neutropenia, leukopenia, and anemia. Treatment-related deaths were uncommon but occurred more frequently in the tiragolumab arm… (3% vs 1%); no new safety signals were identified.
Conclusion
SKYSCRAPER-08 is the first phase III trial to demonstrate a statistically significant survival benefit for anti-TIGIT therapy combined with PD-L1 inhibition and chemotherapy in first-line unresectable or metastatic oesophageal squamous cell carcinoma. The combination of tiragolumab and atezolizumab improved both progression-free and overall survival compared with chemotherapy alone, with an acceptable and predictable safety profile.
These findings support dual checkpoint blockade as a biologically and clinically meaningful strategy in ESCC and provide a strong rationale for further exploration of TIGIT-targeted therapies in gastrointestinal oncology. If confirmed across broader populations and treatment backbones, this approach may represent a promising investigational first-line strategy for patients with high unmet clinical need. These findings were observed in an exclusively Asian population treated with a cisplatin–paclitaxel backbone.
Read full article here.

You can also read about SKYSCRAPER-07 at ESMO 2025: Tiragolumab Plus Atezolizumab After dCRT in Locally Advanced ESCC on OncoDaily.