Fresh from the podium at the ESMO Congress 2025, Dr. Ian Chau (Sutton, United Kingdom) presented the results of SKYSCRAPER-07 trial (NCT04543617), evaluating the combination of tiragolumab (anti-TIGIT) and atezolizumab (anti-PD-L1) as consolidation therapy for patients with unresectable locally advanced esophageal squamous cell carcinoma (ESCC) following definitive chemoradiotherapy (dCRT).
SKYSCRAPER-07 was designed to assess whether dual immune checkpoint blockade could further improve progression-free and overall survival compared to PD-L1 inhibition or placebo alone.
Background
Esophageal squamous cell carcinoma (ESCC) remains a major global health challenge, particularly in Asia, where it accounts for the majority of esophageal cancer cases. For patients with locally advanced, unresectable ESCC, definitive chemoradiotherapy (dCRT) is the established standard of care, offering curative intent through the combination of concurrent chemotherapy and radiation therapy. Despite initial responses, over 50% of patients experience locoregional or distant recurrence, reflecting the aggressive nature of the disease and the limited durability of current treatment approaches.
In recent years, immunotherapy has transformed the management of advanced and metastatic ESCC, demonstrating durable responses and survival benefitsa. This success has prompted investigation of immunotherapy earlier in the treatment course, including as consolidation or maintenance therapy following dCRT, with the goal of preventing relapse and prolonging survival.
The SKYSCRAPER-07 trial was developed to test this strategy by combining tiragolumab, an anti-TIGIT antibody that enhances T-cell activation, with atezolizumab, an anti-PD-L1 checkpoint inhibitor. The study aimed to determine whether this dual blockade could provide synergistic immune activation and improve outcomes compared with atezolizumab or placebo alone in patients who had completed dCRT for unresectable locally advanced ESCC.
Methods
This global, randomized, double-blind, Phase III study enrolled 760 patients with stage II–IVA (selected IVB) locally advanced ESCC who were ineligible for curative surgery. Eligible participants had an ECOG performance status of 0–1 and had completed dCRT.
Patients were randomized 1:1:1 to receive:
- Arm A: Tiragolumab 600 mg IV every 3 weeks + atezolizumab 1200 mg IV every 3 weeks
- Arm B: Atezolizumab 1200 mg IV every 3 weeks + placebo
- Arm C: Double placebo
Treatment continued for up to 17 cycles or until progression or unacceptable toxicity.
Primary endpoints were investigator-assessed progression-free survival (PFS; Arm A vs Arm C), followed hierarchically by overall survival (OS; Arm A vs Arm C) and OS (Arm B vs Arm C).
Results
At the February 18, 2025 data cutoff, 760 patients were randomized (257 in Arm A, 250 in Arm B, 253 in Arm C), with a median follow-up of 25 months. Baseline characteristics were balanced across arms.
Progression-Free Survival (PFS):
- Arm A (tira + atezo): Median PFS 20.8 months (95% CI, 13.9–28.8)
- Arm B (atezo + placebo): Median PFS 29.1 months (95% CI, 19.0–36.3)
- Arm C (placebo): Median PFS 16.6 months (95% CI, 11.2–19.4)
Hazard ratios: Arm A vs C HR 0.82 (95% CI, 0.65–1.03; p=0.0947); Arm B vs C HR 0.74 (95% CI, 0.58–0.93; descriptive p=0.0113)
Overall Survival (OS):
- Arm A: 38.6 months (95% CI, 28.8–NE)
- Arm B: Not reached (95% CI, NE)
- Arm C: 36.4 months (95% CI, 25.8–NE)
Descriptive p-values: Arm A vs C p=0.4772; Arm B vs C p=0.0085
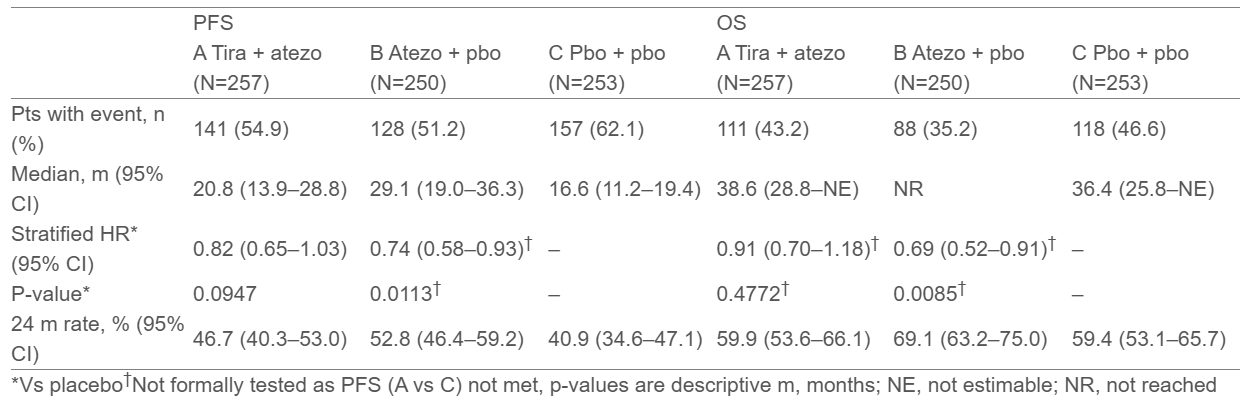
While the study did not meet its primary endpoint (PFS Arm A vs C), atezolizumab monotherapy demonstrated clinically meaningful improvement in both PFS and OS compared to placebo.
Safety
Treatment-related adverse events (AEs) occurred in 74.8% of patients in the tiragolumab + atezolizumab arm, 65.2% in the atezolizumab arm, and 90% in the placebo arm. The most common grade 3–4 AEs included neutropenia and elevated transaminases. Importantly, no new safety signals or unexpected toxicities were identified, and the safety profile was consistent with previous studies of both agents.
Conclusions
The SKYSCRAPER-07 trial did not achieve its primary endpoint of improved progression-free survival for the combination of tiragolumab and atezolizumab versus placebo in patients with unresectable locally advanced ESCC after dCRT.
However, atezolizumab monotherapy demonstrated clinically meaningful prolongation of PFS and OS compared with placebo, supporting continued exploration of PD-L1 inhibition in this setting. Dual TIGIT/PD-L1 blockade remains a promising area of investigation, but further refinement of patient selection may be required to realize its full potential.
You can read the full abstract here.


