At ASCO GI 2026, Denice Kamp presented the results of the LyRiCX randomized phase II trial, evaluating alternative platinum-free or reduced-neurotoxicity chemotherapy backbones, with or without nivolumab, for the first-line treatment of patients with metastatic or unresectable esophagogastric adenocarcinoma.
Title: Liposomal irinotecan, carboplatin or oxaliplatin (LyRICX) with or without nivolumab in the first-line treatment of metastatic or irresectable esophagogastric adenocarcinoma: A randomized phase 2 study.
Authors: Denice Kamp, Merel van Velzen, Rob Kessels, Sebastiaan Siegerink, Anne M. May, Irene V. Hellemond, Theo van Voorthuizen, Ronald Hoekstra, Marco Polee, Sarah Derks, Nadia Haj Mohammad, Hanneke W. van Laarhoven
Background
Oxaliplatin-based regimens remain a standard of care in metastatic or unresectable esophagogastric adenocarcinoma but are frequently associated with cumulative neurotoxicity, negatively affecting quality of life and limiting the feasibility of subsequent therapies. The LyRiCX study was designed to identify the most favorable first-line chemotherapy backbone by balancing efficacy and neurotoxicity, particularly in the context of contemporary immunotherapy use.
Methods and Endpoints
LyRiCX was a multicenter, open-label, randomized phase II trial conducted across 36 medical centers in the Netherlands. Adults with previously untreated, pathologically confirmed HER2-negative metastatic or unresectable esophagogastric adenocarcinoma were enrolled.
Patients were randomized to one of three chemotherapy backbones:
- F-Nal-Iri: nanoliposomal irinotecan, leucovorin, and fluorouracil
- CapCar: capecitabine and carboplatin
- CapOx: capecitabine and oxaliplatin
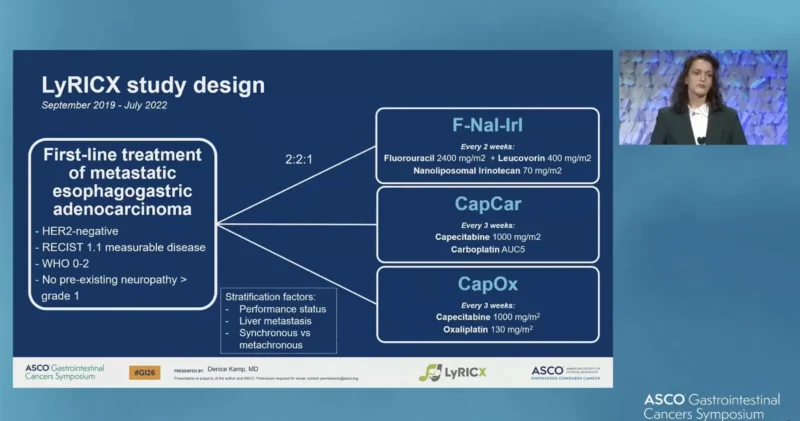
Before approval of nivolumab (until August 2022), patients were randomized in a 2:2:1 ratio to F-Nal-Iri, CapCar, or CapOx. After nivolumab approval, treatment allocation was stratified by PD-L1 Combined Positive Score (CPS):
Patients with CPS <5 or contraindications to nivolumab were randomized to chemotherapy alone (F-Nal-Iri, CapCar, or CapOx; 2:2:1).
Patients with CPS ≥5 were randomized to CapCar or CapOx plus nivolumab (2:1). The primary outcomes were:
- Grade 2–4 neurotoxicity
- Progression-free survival (PFS)
A predefined pick-the-winner strategy was used to identify the most favorable regimen.
Results
Between September 2019 and January 2025, 320 patients were randomized. The median age was 65 years, and 81%of patients were male. Treatment allocation was as follows: F-Nal-Iri (n=83), CapCar (n=157; including 74 with nivolumab), and CapOx (n=80; including 36 with nivolumab). Median PFS follow-up was 24.1 months.
Neurotoxicity
Grade 2–4 neurotoxicity occurred in:
- 0% of patients receiving F-Nal-Iri
- 2.5% of patients receiving CapCar ± nivolumab
- 46.3% of patients receiving CapOx ± nivolumab
There was no significant difference in neurotoxicity between CapCar and F-Nal-Iri (p=0.301). In contrast, neurotoxicity rates were significantly higher with CapOx compared with both CapCar and F-Nal-Iri (both p<0.001). No other toxicity patterns emerged that outweighed the marked differences observed in neurotoxicity across treatment arms.
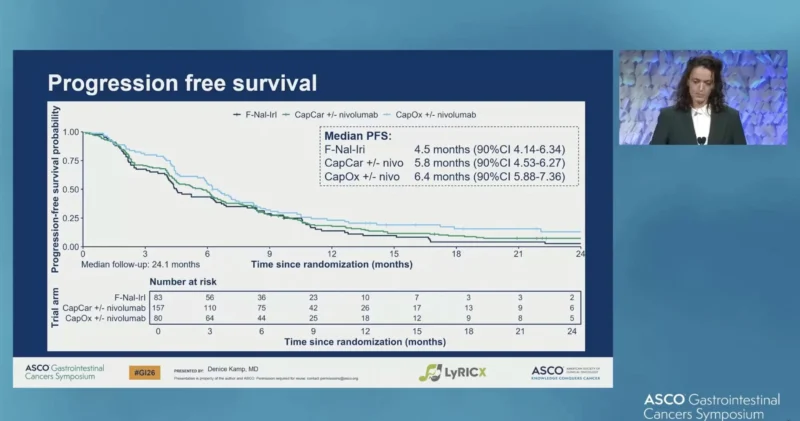
Progression-Free Survival
Median PFS was:
- 4.5 months (90% CI 4.14–6.34) with F-Nal-Iri
- 5.8 months (90% CI 4.53–6.27) with CapCar ± nivolumab
- 6.4 months (90% CI 5.88–7.36) with CapOx ± nivolumab
Adverse Events
Overall, safety profiles were broadly comparable across treatment arms, with no new or unexpected safety signals observed. Apart from neurotoxicity, rates of selected grade 3–4 adverse events were generally similar between regimens.
With respect to specific toxicities, anemia occurred across all treatment arms, with higher rates observed in the CapCar ± nivolumab arm compared with F-Nal-Iri and CapOx ± nivolumab. Importantly, the main driver of differential toxicity was neurotoxicity, which was markedly higher with CapOx ± nivolumab compared with CapCar ± nivolumab and F-Nal-Iri.
Conclusion
The LyRiCX trial demonstrates that CapCar and F-Nal-Iri markedly reduce grade 2–4 neurotoxicity compared with CapOx, while achieving similar progression-free survival and without an excess of other toxicities. Given its favorable neurotoxicity profile, ease of administration without the need for a central venous line, and relatively low cost due to off-patent agents, CapCar emerged as the most favorable first-line chemotherapy backbone for patients with metastatic or unresectable esophagogastric adenocarcinoma.
Read Full abstract here.

You can also read about CRITICS-II Trial at ASCO GI: Total Neoadjuvant Therapy in Resectable Gastric Cancer on OncoDaily.


