CRITICS-II Trial, presented fresh from the ASCO Gastrointestinal Cancers Symposium, is a multicenter randomized phase II trial evaluating three preoperative-only treatment strategies in patients with resectable gastric cancer. The study compared neoadjuvant chemotherapy alone, chemotherapy followed by chemoradiotherapy, and chemoradiotherapy alone, with the aim of identifying the most effective and feasible preoperative regimen while omitting adjuvant treatment.
Background
Radical surgery remains the cornerstone of the cure for non-metastatic, resectable gastric cancer, yet long-term outcomes remain suboptimal. Several evidence-based perioperative strategies have been developed to improve survival, including perioperative chemotherapy, postoperative chemoradiotherapy, and postoperative chemotherapy. However, compliance with postoperative therapy is consistently low, particularly after major gastric surgery.
Preoperative treatment offers several potential advantages, including improved treatment adherence, tumor downstaging, and increased rates of complete (R0) resection. Against this background, the CRITICS-II trial was designed to identify the optimal preoperative-only treatment strategy while omitting adjuvant therapy, with the goal of selecting the most effective and feasible regimen for further phase III development.
Study Design and Methods
CRITICS-II Trial was a multicenter, randomized phase II trial conducted across 16 centers in the Netherlands. Patients with clinical stage IB–IIIC (TNM 8th edition) resectable gastric or gastroesophageal junction adenocarcinoma were eligible, provided there were no distant metastases and staging laparoscopy was negative. Tumors had to be located in the stomach or at the gastroesophageal junction, with the bulk of the disease in the stomach. Eligible patients had a WHO performance status 0–1 and were aged ≥18 years.
Patients were randomized to one of three preoperative-only treatment arms:
- Arm 1: Four cycles of docetaxel, oxaliplatin, and capecitabine (DOC)
- Arm 2: Two cycles of DOC followed by chemoradiotherapy (45 Gy in 25 fractions with concurrent weekly paclitaxel and carboplatin)
- Arm 3: Chemoradiotherapy alone (45 Gy in 25 fractions with concurrent paclitaxel and carboplatin)
All patients proceeded to surgery consisting of total or subtotal gastrectomy with extended lymphadenectomy (D1+ or D2), with removal of at least 15 lymph nodes. Tumor tissue, blood samples, and health-related quality-of-life data were collected before, during, and after treatment. Randomization was stratified by center and histological subtype.
The primary endpoint was 1-year event-free survival (EFS), using a pick-the-winner design in which ≤60% was considered insufficiently active and ≥75% sufficiently active. Secondary endpoints included toxicity, compliance, surgical outcomes, R0 resection rate, pathological response, overall survival (OS), postoperative complications, and quality of life.
Results of CRITICS-II Trial
Between 2017 and 2024, 201 patients were randomized, with a median follow-up of 40.4 months. Approximately 69 patients per arm were planned, consistent with the trial’s statistical assumptions. Baseline characteristics were well balanced across treatment groups, although slightly more gastroesophageal junction tumors were observed in the chemotherapy-containing arms.
Safety and Toxicity
Preoperative toxicity was acceptable across all arms. Grade 3–4 adverse events occurred in 52% of patients receiving chemotherapy alone, 53% in the chemotherapy plus chemoradiotherapy arm, and 43% in the chemoradiotherapy-only arm, indicating the lowest severe toxicity burden with chemoradiotherapy alone.
Surgical Outcomes
Surgical resection was performed in most patients across all treatment arms. Curative-intent surgery was undertaken in 60 patients in the chemotherapy-alone arm, 58 patients in the chemotherapy plus chemoradiotherapy arm, and 63 patients in the chemoradiotherapy-alone arm
Efficacy Outcomes
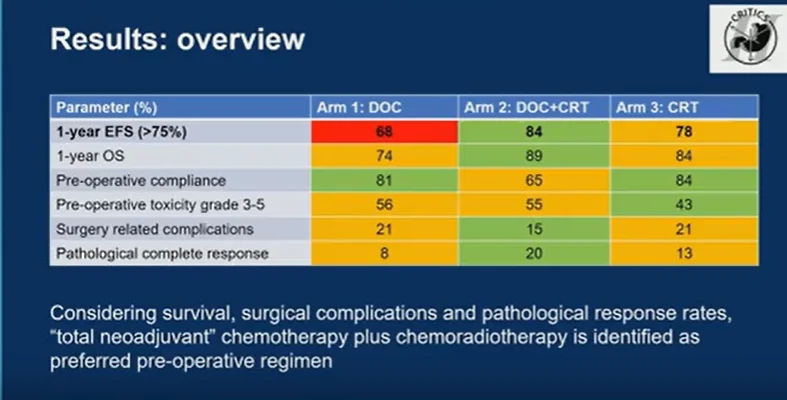
The primary endpoint of 1-year event-free survival clearly distinguished the treatment strategies. Chemotherapy alone achieved a 1-year EFS of 68%, failing to meet the predefined efficacy threshold. In contrast, chemotherapy followed by chemoradiotherapy achieved the highest 1-year EFS at 84%, while chemoradiotherapy alone reached 78%, both meeting criteria for sufficient activity.
Overall survival at 1 year followed a similar pattern, with rates of 74% for chemotherapy alone, 89% for chemotherapy plus chemoradiotherapy, and 84% for chemoradiotherapy alone.
Clinical Implications
Taken together, the results demonstrate that preoperative chemotherapy alone was inferior, failing to meet the EFS efficacy threshold and showing the lowest survival outcomes. Both chemotherapy followed by chemoradiotherapy and chemoradiotherapy alone were sufficiently effective, but important differences emerged when considering toxicity, surgical complications, and pathological response.
While chemoradiotherapy alone was associated with lower preoperative toxicity and higher compliance, chemotherapy followed by chemoradiotherapy consistently showed the most favorable balance, with the highest event-free and overall survival, the highest pathological complete response rate, and the lowest rate of surgery-related complications.
Conclusion
CRITICS-II Trial identifies total neoadjuvant therapy consisting of chemotherapy followed by chemoradiotherapy as the preferred preoperative strategy for patients with resectable gastric cancer when adjuvant treatment is omitted. This approach appears feasible, effective, and safe, and emerges as the leading candidate for further evaluation, particularly in the context of organ-sparing treatment strategies.

For more information, click here.


