The BRAFV600E mutation, present in around 8–10% of colorectal cancers, is associated with poor prognosis, right-sided tumor predominance, and resistance to standard chemotherapy. While microsatellite instability-high tumors benefit from immunotherapy, the majority of BRAFV600E cases are microsatellite stable (MSS) and do not respond to PD-1 inhibitors alone. Encorafenib plus cetuximab has provided modest survival benefit, but durable disease control remains limited.
This trial evaluated whether the addition of nivolumab could improve outcomes for patients with MSS BRAFV600E metastatic colorectal cancer (mCRC).
What is the Standard Treatment for BRAFV600E-Mutated Metastatic CRC?
According to NCCN Guidelines Version 4.2025, patients with BRAFV600E-mutated metastatic colorectal cancer may receive encorafenib in combination with cetuximab (or panitumumab), with or without FOLFOX, as part of recommended initial therapy. In the second-line and subsequent settings, the NCCN likewise recommends encorafenib plus cetuximab (or panitumumab), with the option to add FOLFOX (category 2B). This positions the targeted doublet as the backbone of care across multiple lines of therapy once the mutation is identified.
Previously, the BEACON trial established this doublet regimen as a new standard of care. Updated survival results, published in Journal of Clinical Oncology in 2021, reported a median overall survival of 9.3 months with encorafenib plus cetuximab compared with 5.9 months for standard chemotherapy, along with an objective response rate close to 20%. These findings confirmed the superiority of the targeted regimen and laid the foundation for its inclusion in current international guidelines.
Trial Design
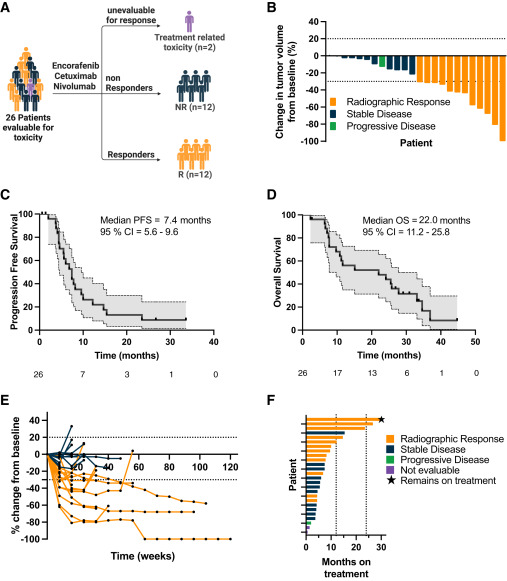
This single-institution, open-label phase 1/2 study enrolled 26 patients with MSS BRAFV600E mCRC. The phase 1 portion established the recommended phase 2 dose with no dose-limiting toxicities.
Patients received:
- Encorafenib (BRAF inhibitor)
- Cetuximab (anti-EGFR)
- Nivolumab (anti-PD-1)
Treatment continued until progression or unacceptable toxicity. Serial biopsies and extracellular vesicle RNA (evRNA) from plasma were analyzed for immune and MAPK pathway activation signatures.
Read more about Opdivo (Nivolumab): Uses in Cancer, Side Effects, Dosages, Expectations on OncoDaily.
Endpoints
- Primary endpoint: Overall Response Rate (ORR)
- Secondary endpoints: Progression-Free Survival (PFS), Overall Survival (OS), Duration of Response (DoR), Safety
- Exploratory endpoints: Transcriptomic correlates of response using tumor tissue and evRNA.
Results
Among 24 evaluable patients, the ORR was 50% (95% CI: 29–71), with one complete response and 11 partial responses. The disease control rate reached 96%, and the median duration of response was 7.7 months. In the intention-to-treat population, the ORR was 46%. Median PFS was 7.4 months (95% CI: 5.6–9.6), while median OS was 22 months (95% CI: 11.2–25.8). Two patients remained progression-free for more than two years. Responses were more common in left-sided tumors than right-sided, and responders showed markedly improved PFS and OS compared to non-responders.
Biomarker analyses demonstrated that responders had enrichment of immune activation and p38 MAPK signaling, while non-responders exhibited complement activation and higher metabolic activity signatures. evRNA profiling confirmed these findings, showing that responders developed dynamic increases in interferon gamma signatures and decreases in MAPK signaling over the course of treatment.
Safety
No dose-limiting toxicities were observed. Grade ≥3 adverse events occurred in 23% of patients, most commonly asymptomatic elevations in lipase and amylase, along with individual cases of colitis, rash, and myositis/myocarditis. Common all-grade adverse events included headache, nausea, arthralgia, anemia, rash, pruritus, and infusion reactions. Dermatologic toxicities such as keratoacanthomas and squamous cell carcinomas occurred in six patients but were effectively managed with local treatment.
The results were published in Cancer Cell.
Conclusion
The combination of encorafenib, cetuximab, and nivolumab produced substantial clinical activity in patients with MSS BRAFV600E mCRC, a subgroup with historically poor outcomes. With an ORR of 50%, median PFS of 7.4 months, and median OS of 22 months, the regimen compares favorably with encorafenib plus cetuximab alone. Biomarker findings suggest that immune activation and MAPK modulation may underpin therapeutic benefit, while complement activation and metabolic pathways are associated with resistance. These results support ongoing randomized evaluation in the SWOG 2107 phase 2 trial and highlight the potential of combining targeted therapy with immunotherapy in this difficult-to-treat population.