A novel bispecific T-cell engager Obrixtamig, targeting DLL3 and CD3, has demonstrated encouraging antitumor activity and a manageable safety profile in patients with advanced or metastatic DLL3-positive small cell lung cancer (SCLC), extrapulmonary neuroendocrine carcinoma (epNEC), and large-cell neuroendocrine carcinoma of the lung (LCNEC-L). Findings from the Phase Ia/Ib dose-escalation/dose-expansion study (NCT04429087) support further clinical development, particularly for patients who have exhausted standard treatment options.
Study Design and Patient Population
This multicenter, open-label, phase Ia/Ib trial enrolled adult patients with locally advanced or metastatic SCLC, epNEC, LCNEC-L, or other small cell carcinoma variants. All patients had DLL3-positive tumors, confirmed centrally using the Ventana DLL3 (SP347) assay. Eligibility required prior progression after at least one line of platinum-based therapy.
Patients were recruited across Germany, Japan, Spain, and the United States. The primary objectives of the phase Ia portion were to determine the maximum tolerated dose (MTD) and assess dose-limiting toxicities (DLTs). Key secondary endpoints included pharmacokinetics (PK) and objective response rate (ORR) per RECIST v1.1 by investigator assessment.
Dosing Regimens and Safety Management
Four intravenous obrixtamig regimens were evaluated:
- Regimen A: Fixed dose every 3 weeks
- Regimen B1: Weekly fixed dose (Days 1, 8, 15 of each cycle)
- Regimen B2: Two weekly step-up doses, then fixed weekly
- Regimen B3: Three step-up doses weekly, then fixed weekly x3, followed by every 3 weeks
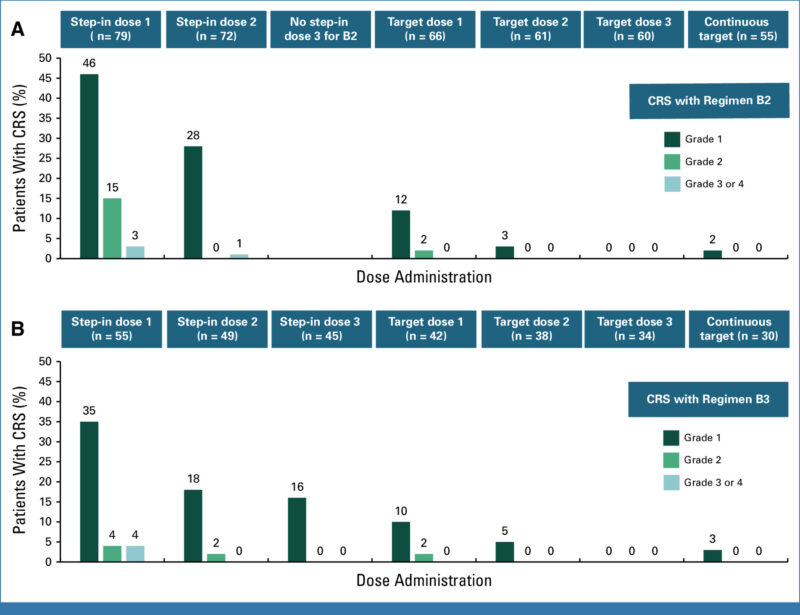
Due to the known risk of cytokine release syndrome (CRS) associated with TCEs, step-up dosing was introduced in Regimens B2 and B3. Premedication protocols included corticosteroids, antihistamines, and acetaminophen to reduce CRS and infusion-related reactions (IRRs).
Efficacy Outcomes Across Tumor Types
As of February 21, 2024, a total of 168 patients received obrixtamig. Of these, 72% had received two or more prior lines of anticancer therapy, and 51% had been previously treated with anti–PD-1/PD-L1 therapy. Seven dose-limiting toxicities (DLTs) were observed during MTD evaluation—one in Regimen A and six in Regimen B2. The maximum tolerated dose (MTD) was not reached.
The most common treatment-related adverse event was cytokine release syndrome (CRS), occurring in 57% of patients (any grade) and 3% of patients (grade ≥3). Most CRS events occurred early and were reversible. Across all doses, regimens, and tumor types, the overall response rate (ORR) was 23% (95% CI, 17.4%–30.2%), with a median duration of response (DoR) of 8.5 months (95% CI, 6.2–not reached). The 6-month DoR rate was 70% (95% CI, 53%–88%).
Among patients in Regimens B2 and B3 who received the minimum effective dose (≥90 μg/kg once weekly or every 3 weeks), the ORR was 28% (95% CI, 20.7%–35.9%). Response rates by tumor type within these regimens were 21% (95% CI, 12.9%–33.1%) for small cell lung cancer (SCLC), 27% (95% CI, 17.4%–39.6%) for extrapulmonary neuroendocrine carcinomas (epNECs), and 70% (95% CI, 39.7%–89.2%) for large-cell neuroendocrine carcinoma of the lung (LCNEC-L).
You can read the Full Article on Journal of Clinical Oncology.
How Obrixtamig Works? Targeting DLL3 in Aggressive Neuroendocrine Tumors
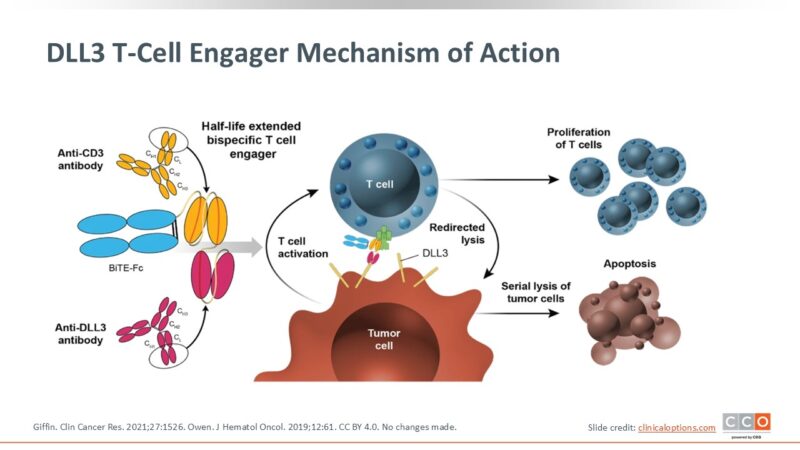
Obrixtamig (also known as HPN328) is a novel T-cell engager designed to harness the body’s immune system to fight cancer. It targets a specific protein called delta-like ligand 3 (DLL3), which is abnormally expressed on the surface of certain aggressive neuroendocrine tumors—including small cell lung cancer (SCLC), large-cell neuroendocrine carcinoma (LCNEC), and extrapulmonary neuroendocrine carcinomas (epNEC).
DLL3 is typically absent or minimally expressed in normal tissues, but it is overexpressed in more than 80% of SCLC tumors, making it an ideal target for therapy. Obrixtamig is designed to bind both DLL3 on tumor cells and CD3 on T cells, effectively creating a bridge between the cancer and the immune system.
Once obrixtamig binds to DLL3 on a cancer cell and CD3 on a T cell, it activates the T cell, leading to the release of cytotoxic proteins that directly kill the tumor cell. This mechanism allows for targeted killing of DLL3-positive cancer cells, while sparing healthy tissues. By leveraging this tri-specific antibody platform, obrixtamig shows promise as a next-generation immunotherapy for patients with DLL3-positive, hard-to-treat neuroendocrine tumors.
source: clinicaloptions.com
Future Directions and Ongoing Trials
Based on favorable pharmacokinetics, safety, and early efficacy, step-up dosing regimens—particularly Regimen B3—have been selected for further clinical development. Multiple trials evaluating obrixtamig are now underway:
- NCT05879978: Obrixtamig in combination with the PD-1 inhibitor ezabenlimab for DLL3-positive SCLC and NECs
- NCT06132113: Obrixtamig with standard first-line chemotherapy in DLL3-positive NECs
- NCT05882058: A Phase II trial exploring dose selection in pretreated SCLC, epNEC, or LCNEC-L
- NCT05990738 and NCT06077500: Combinations with single-agent chemotherapy or frontline regimens in advanced SCLC
Context with Other DLL3-Directed Therapies
When compared with emerging DLL3-targeted agents such as Tarlatamab and MK-6070, obrixtamig’s efficacy and tolerability are broadly consistent, with CRS and dysgeusia representing common class effects. However, important distinctions in trial design exist. Notably, this trial did not require baseline CNS imaging, allowing for enrollment of patients with asymptomatic brain metastases—a real-world population often excluded from early-phase studies.
Additionally, while other studies have evaluated DLL3-targeted therapies primarily in SCLC, this trial is among the first to explore activity across multiple tumor types, including epNEC and LCNEC-L. The response rate observed in LCNEC-L, a group with very limited treatment options in the second-line setting, highlights a particularly compelling signal that warrants further investigation.
Conclusion
Obrixtamig demonstrated a favorable safety profile and encouraging antitumor activity across three DLL3-expressing high-grade neuroendocrine tumor types. The use of step-up dosing strategies significantly reduced CRS and improved tolerability, while efficacy signals—particularly in LCNEC-L—exceeded expectations for second-line settings. With multiple trials now expanding into combination and frontline regimens, obrixtamig may offer a valuable new approach for patients with aggressive neuroendocrine carcinomas who have few therapeutic options.


