Advanced, well-differentiated pancreatic neuroendocrine tumors (panNETs) represent a biologically heterogeneous disease where long-term disease control is achievable, yet cure remains elusive. Over the past decade, multiple systemic options—including everolimus, streptozotocin-based chemotherapy, CAPTEM, tyrosine kinase inhibitors, and PRRT—have expanded the therapeutic landscape. Despite these advances, a central unresolved question in daily practice has remained: what is the optimal sequence of available therapies?
Everolimus and streptozotocin plus 5-fluorouracil (STZ/5-FU) are both established standards in advanced panNETs, but until now, no prospective randomized trial had directly compared their sequencing. The international phase III SEQTOR-GETNE trial was designed to address this critical gap.
You can read about Pancreatic Neuroendocrine Tumors Overview: Comprehensive 2025 Insights on OncoDaily.
SEQTOR study Methods and Endpoints
SEQTOR was an international, open-label, randomized, crossover phase III trial conducted across eight European countries. Adults with unresectable or metastatic, well-differentiated (WHO grade 1–2) panNETs and ECOG performance status 0–2 were eligible. Patients were required to have radiologic progression within 12 months, although selected treatment-naive patients could also be included.
Patients were randomized 1:1 to one of two strategies:
- Everolimus 10 mg daily followed by STZ/5-FU at progression
- STZ/5-FU followed by everolimus at progression
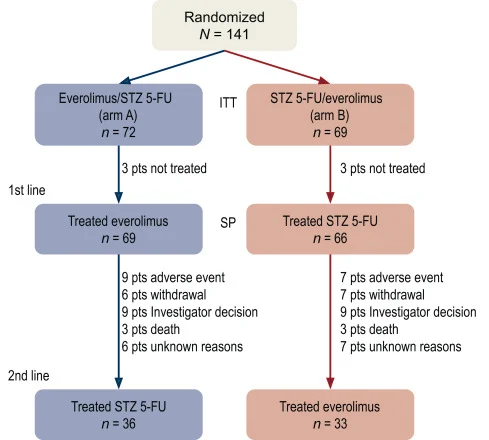
Crossover to the alternate treatment at progression was planned for all patients and was implemented in about half of the cohort. The study was initially designed with combined PFS across both lines as the primary endpoint, but due to slow accrual and long survival, the protocol was amended, and 12-month PFS after first-line therapy (12-month PFS1) became the primary endpoint.
Key secondary endpoints included:
- PFS to first and second treatment (PFS1 and PFS2)
- Overall response rate (ORR) and duration of response
- Overall survival (OS)
- Safety and quality of life
Tumor response was assessed locally and by blinded independent central review using RECIST 1.0.
Results of SEQTOR study
The final analysis, published in ESMO Open in December 2025, included 141 randomized patients, with 135 receiving at least one dose of study treatment. Baseline characteristics were well balanced, with over 90% of patients presenting with metastatic disease and the majority having grade 2 tumors.
Primary endpoint
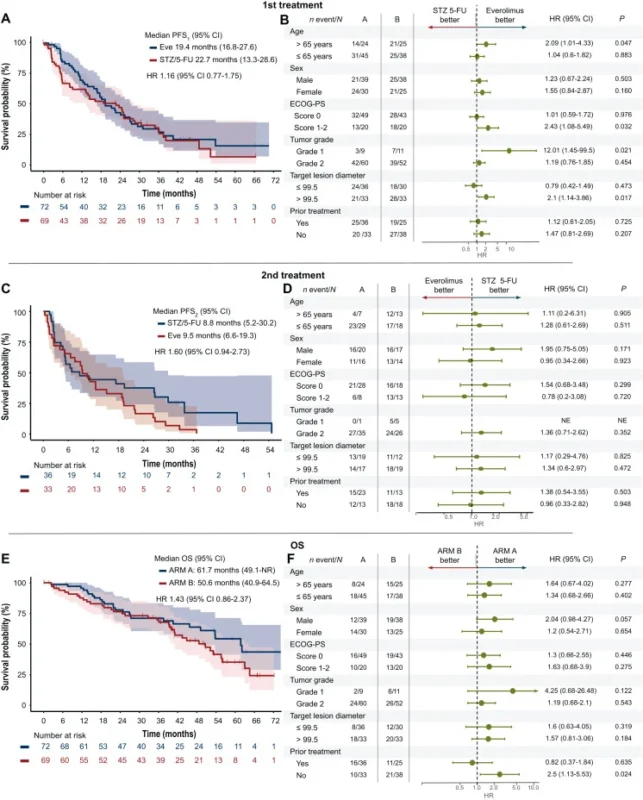
No statistically significant difference in 12-month PFS1 was observed between treatment sequences. The 12-month PFS1 rate was 71.4% with upfront everolimus and 61.8% with upfront STZ/5-FU. Median first-line PFS was similarly prolonged at 19.4 months and 22.7 months, respectively.
Across both treatment lines
- Median PFS2 was around 9–10 months with either sequence, with no meaningful difference between arms.
- Combined PFS1+2 exceeded 30 months in both arms
- Overall survival was comparable, with median OS of 61.7 months for everolimus-first and 50.6 months for STZ/5-FU-first
Tumor response
A clear difference emerged in ORR
- STZ/5-FU achieved significantly higher response rates than everolimus in the first line, and numerically higher response rates in the second line.
- ORR was ~30% with STZ/5-FU versus ~10% with everolimus.
- Responses to STZ/5-FU were highly durable, with median duration exceeding two years in first line
Exploratory subgroup analyses suggested that younger patients, those with ECOG 0, and patients with grade 2 tumors derived particularly high response benefit from STZ/5-FU. Conversely, signals of better survival with upfront everolimus were observed in older patients, those with ECOG 1–2, and grade 1 tumors, although these findings were hypothesis-generating.
Regarding safety, toxicity profiles reflected known class effects. Everolimus was associated with higher rates of mucositis, skin toxicity, hyperglycemia, edema, and pneumonitis, and required significantly more dose reductions and treatment interruptions. STZ/5-FU was linked primarily to gastrointestinal symptoms and mild renal impairment, most often low grade. No toxic deaths occurred.
Quality-of-life scores declined gradually over time in both arms, and no major overall differences emerged between sequences, although the everolimus-first group started with slightly worse baseline QoL and showed a more pronounced decline in physical functioning over time.
What Are Streptozotocin and Everolimus, and How Do They Work?
Both treatment strategies evaluated in the SEQTOR trial—Streptozotocin plus 5-fluorouracil and Everolimus—represent fundamentally different therapeutic approaches in pancreatic neuroendocrine tumors (panNETs), targeting distinct biological vulnerabilities.
Streptozotocin
Streptozotocin is a classic cytotoxic alkylating agent with a unique biological affinity for pancreatic tissue and pancreatic neuroendocrine tumors (panNETs). Structurally similar to glucose, streptozotocin enters tumor cells through the glucose transporter GLUT2, which is highly expressed in pancreatic beta cells and panNETs. This selective uptake explains its preferential antitumor activity in this disease.
Once internalized, streptozotocin induces DNA alkylation and double-strand breaks, irreversibly disrupting DNA replication and triggering tumor cell death. In clinical practice, it is most commonly combined with 5-fluorouracil (5-FU), which inhibits thymidylate synthase and further impairs DNA synthesis. This combination results in synergistic cytotoxicity, translating into higher objective response rates and meaningful tumor shrinkage, particularly valuable in patients with bulky or symptomatic disease requiring cytoreduction.
Everolimus
Everolimus is a targeted oral anticancer agent that acts as a selective inhibitor of the mTOR (mammalian target of rapamycin) signaling pathway. The mTOR pathway is a central regulator of cancer cell growth, metabolism, survival, and angiogenesis, and is frequently overactivated in neuroendocrine tumors.
By blocking mTOR activity, everolimus exerts a primarily cytostatic effect, suppressing tumor cell proliferation, reducing protein synthesis, and inhibiting tumor angiogenesis. Unlike streptozotocin, everolimus rarely induces rapid tumor shrinkage but is highly effective at stabilizing disease and prolonging progression-free survival, particularly in slow-growing, well-differentiated panNETs. Its oral administration and long-term disease control profile make it a cornerstone therapy for chronic management of advanced neuroendocrine tumors.
Conclusion
SEQTOR is the first randomized phase III study to prospectively compare the sequence of two standard therapies in advanced panNETs. While the trial could not define a single superior sequence, it delivers several clinically important insights.
- Everolimus and STZ/5-FU provide equivalent long-term disease control and overall survival
- STZ/5-FU consistently produces higher and more durable tumor response rates
- Everolimus remains an effective oral option for patients in whom cytoreduction is not urgently required
These data firmly support a personalized treatment strategy in advanced panNETs. Tumor grade, growth kinetics, patient fitness, comorbidities, and the clinical need for cytoreduction should guide first-line selection—rather than a fixed sequencing algorithm. In the modern era, where CAPTEM and PRRT further expand therapeutic options, SEQTOR provides a critical evidence base to individualize long-term management for patients with pancreatic neuroendocrine tumors.
Read full article here.