Oncolytic viral immunotherapies represent an emerging class of cancer therapeutics that combine direct tumor lysis with immune system activation. RP1 (vusolimogene oderparepvec), developed by Replimune Inc., is an engineered strain of herpes simplex virus type 1 (HSV-1) designed to replicate selectively in tumor tissue, induce immunogenic cell death, and enhance systemic antitumor immunity.
In October 2025, the U.S. Food and Drug Administration (FDA) accepted Replimune’s resubmission of the Biologics License Application (BLA) for RP1 in combination with nivolumab (Opdivo®) for patients with advanced, PD-1–refractory melanoma, with a regulatory decision expected by April 2026.
Mechanism of Action
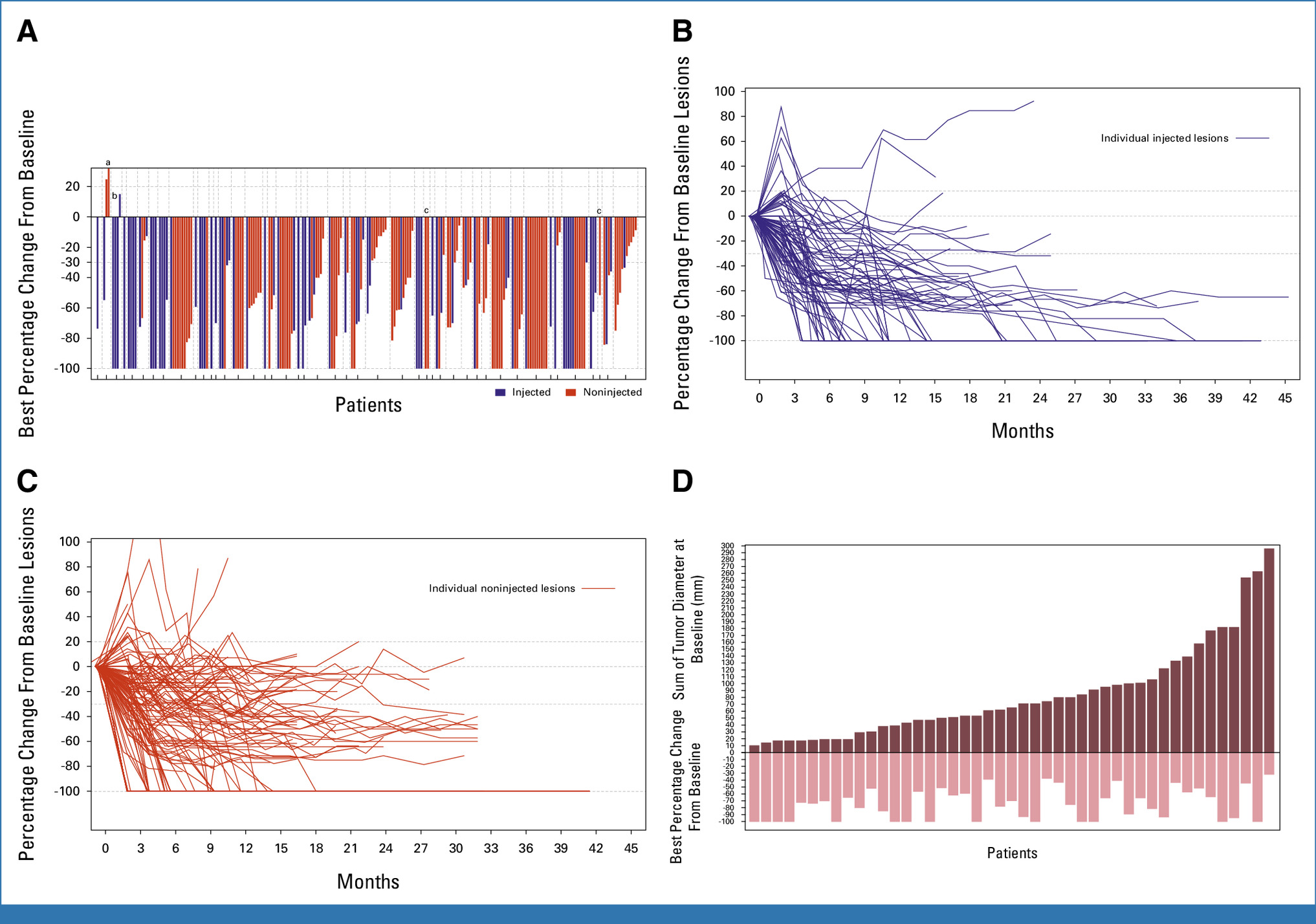
RP1 incorporates several genetic modifications to augment its oncolytic and immune-stimulatory activity. Deletion of the ICP34.5 and ICP47 genes allows selective replication in tumor cells while enhancing antigen presentation and immune recognition. The virus expresses GM-CSF, which promotes dendritic cell recruitment and T-cell priming. When administered intratumorally and combined with PD-1 blockade (nivolumab), RP1 facilitates a dual mechanism—direct tumor destruction and systemic immune activation against metastatic lesions, including non-injected sites.
Clinical Development
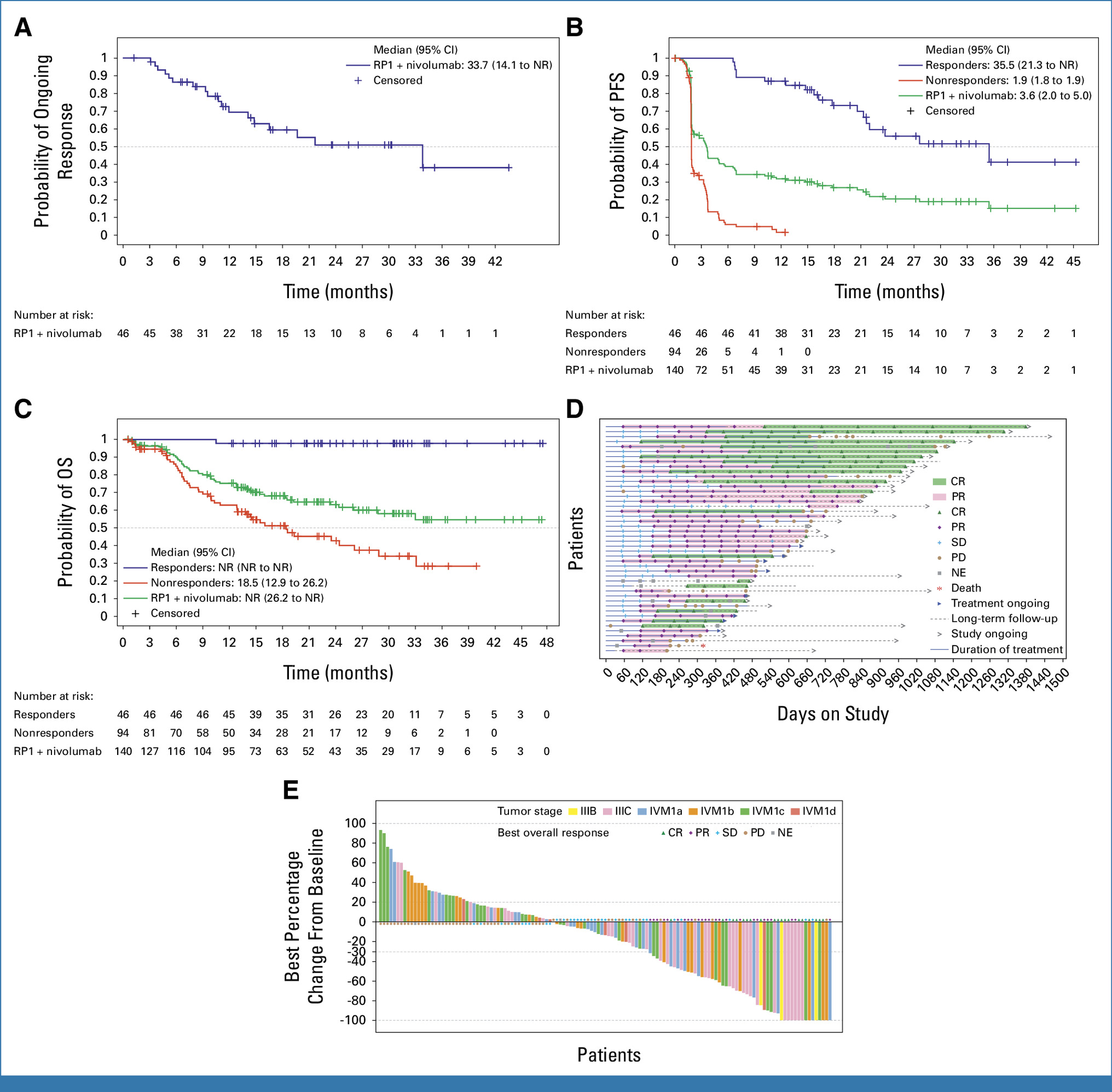
The current resubmission is supported by data from the Phase I/II IGNYTE trial, which enrolled 140 patients with advanced or metastatic melanoma who had previously progressed on PD-1 inhibitors. Patients received RP1 intratumorally in combination with intravenous nivolumab until disease progression or unacceptable toxicity. The objective response rate (ORR) was 32.9%, demonstrating durable and systemic responses across injected and non-injected lesions.
The safety profile was consistent with known effects of oncolytic viruses and immune checkpoint inhibitors, with the majority of adverse events being grade 1–2 (flu-like symptoms, injection-site reactions, fatigue). No new safety signals were observed.
Regulatory Context
In July 2025, the FDA issued a Complete Response Letter (CRL), stating that the IGNYTE trial was “not adequate and well-controlled” and that its efficacy outcomes were “difficult to interpret due to heterogeneity of the patient population.” The decision generated substantial debate within the oncology community regarding regulatory flexibility for therapies targeting small, refractory populations.
In response, Replimune submitted a comprehensive resubmission including additional analyses, subgroup data, and methodological clarifications addressing the FDA’s concerns. The agency has now determined that the submission constitutes a complete response to the prior CRL.
Scientific Significance
The clinical and biological rationale for RP1 is robust. Oncolytic viruses have the capacity to transform immunologically “cold” tumors into “hot” ones by increasing tumor antigen visibility, inflammatory cytokine release, and intratumoral immune cell infiltration. The IGNYTE findings demonstrate that combining RP1 with PD-1 blockade can restore responsiveness in checkpoint inhibitor–refractory melanoma, a population with limited therapeutic options and poor prognosis.
This represents an important proof of concept for oncolytic–checkpoint inhibitor synergy, paralleling mechanisms observed in preclinical models and early clinical studies with other viral immunotherapy platforms.
Implications and Future Directions
If approved, RP1 would be the first oncolytic immunotherapy combined with PD-1 blockade to receive FDA authorization for melanoma. The therapy’s potential to extend survival and induce durable responses in PD-1–resistant disease could redefine the role of viral immunotherapy in immuno-oncology.
The acceptance of the resubmission indicates the FDA’s willingness to re-examine novel immunotherapies with complex trial designs when supported by additional evidence. Ongoing and planned studies are expected to further elucidate the predictive biomarkers of response to RP1, including tumor immune infiltration, viral replication kinetics, and interferon signaling profiles.