Immune checkpoint inhibitors have reshaped first-line therapy for advanced gastroesophageal cancers, but they also complicate the way clinical benefit is measured. Overall survival (OS) remains the ultimate endpoint, yet its long follow-up requirements make it challenging to evaluate new regimens built around immunotherapy. This has renewed interest in whether progression-free survival (PFS), a much earlier readout, can reliably act as a surrogate for OS—both in gastroesophageal adenocarcinoma (GEA) and esophageal squamous cell carcinoma (ESCC), and across clinically relevant PD-L1 subgroups.
To answer this, investigators performed a trial-level meta-analysis in the immunotherapy era, synthesizing evidence from 18 randomized phase III trials.
Methods
A PRISMA-guided systematic review identified first-line phase III RCTs evaluating ICIs with or without chemotherapy in advanced GEA or ESCC (search cutoff: June 30, 2025). Trials were included if they reported hazard ratios (HRs) for both OS and PFS; studies adding further investigational agents (except trastuzumab for HER2-positive disease) were excluded.
Data were collected from full publications and updated abstracts. Eighteen RCTs were eligible:
- GEA: KEYNOTE-062, CHECKMATE-649, ATTRACTION-4, KEYNOTE-590 (GEA subgroup), ORIENT-16, RATIONALE-305, KEYNOTE-811, KEYNOTE-859, GEMSTONE-303, COMPASSION-15
- ESCC: KEYNOTE-590 (ESCC subgroup), CHECKMATE-648, ORIENT-15, JUPITER-06, ESCORT-1ST, RATIONALE-306, ASTRUM-007, GEMSTONE-304, SKYSCRAPER-08
These 18 studies correspond to the trials included in the PRISMA flow diagram of the original meta-analysis. Trial-level surrogacy was assessed using Spearman correlation (R) and weighted linear regression (R²), modelling the treatment effect on PFS as a function of the treatment effect on OS at the trial level. Analyses were conducted not only in the overall populations but also across clinically relevant PD-L1 groups: CPS ≥1, CPS ≥5, CPS <1 for GEA, and CPS ≥10 and TPS ≥1% for ESCC.
When correlations were statistically meaningful, a surrogate threshold effect (STE) was calculated—the degree of PFS benefit required to reliably predict an OS benefit. A leave-one-out validation assessed the robustness of each model.

You can also read about COMPASSION-15 Trial at ESMO 2025: Cado Plus Chemotherapy Extends Survival in HER2-Negative Gastric Cancer on OncoDaily.
Results
Before discussing disease-specific findings, it is important to note that the surrogacy signal was not uniform across all tumor types.
GEA and ESCC demonstrated fundamentally different biological behaviors under immunotherapy:
- In GEA, improvements in early tumor control often translated into longer survival.
- In ESCC, OS benefits were frequently driven by a minority of long-term responders, producing survival tails that PFS could not capture.
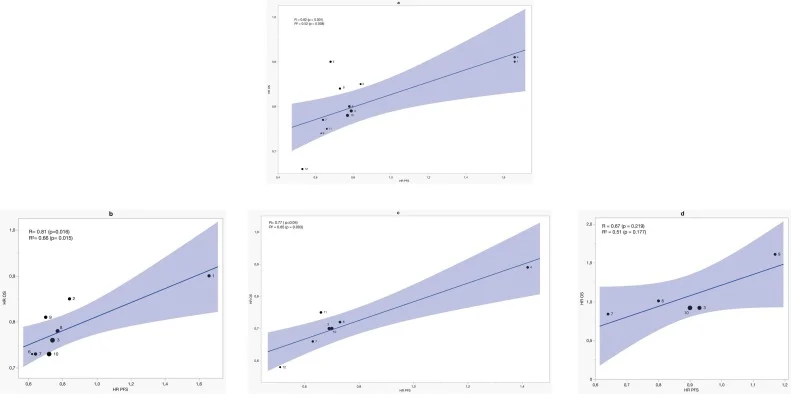
GEA Group
Across ten GEA trials, PFS demonstrated a moderate correlation with OS overall (R = 0.82; R² = 0.52). This relationship was not perfect, but it reflected a consistent pattern: ICI-driven improvements in early tumor control tended to correspond to improvements in survival.
The association strengthened as PD-L1 expression increased.
In CPS ≥1 tumors, the correlation became more pronounced (R = 0.81; R² = 0.66), suggesting that even modest levels of PD-L1 expression support a link between early and long-term outcomes.
The most compelling findings emerged in CPS ≥5, where PFS was a strong surrogate for OS. Here, treatment effects on PFS explained nearly all of the variation in OS across trials (R = 0.77; R² = 0.85). The STE was 0.51 in the overall GEA population, 0.52 in CPS ≥1, and rose to 0.64 in CPS ≥5—meaning that a PFS HR below ~0.64 strongly predicts OS benefit in PD-L1–high disease.
This pattern mirrors clinical practice, where PD-L1–high tumors often exhibit deeper, more durable immunotherapy responses, allowing PFS and OS to move in parallel.
In contrast, PD-L1–negative GEA (CPS <1) showed only moderate but non-significant surrogacy. Correlations were not statistically significant (R = 0.67, p = 0.219), and cross-validation errors were high, indicating an unreliable model. In this subgroup, PFS alone should not be relied on to predict OS, and OS remains essential for interpreting clinical benefit.
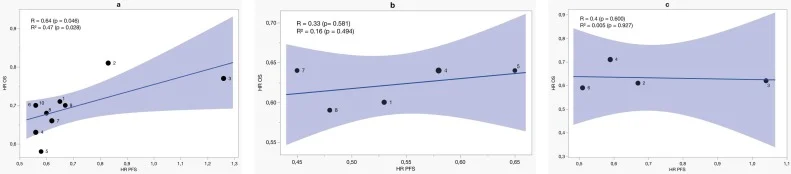
ESCC Group
The picture was markedly different in ESCC. Across nine ESCC trials, the correlation appeared modest when considering R (0.64), but surrogacy remained weak based on the R² value (0.47). Even when immunotherapy improved OS substantially, PFS often changed very little.
PD-L1 enrichment did not rescue this relationship.
- In CPS ≥10, correlations were low and non-significant (R = 0.33; R² = 0.16).
- In TPS ≥1%, surrogacy almost disappeared entirely (R² = 0.005).
These findings reflect a defining feature of ESCC immunotherapy: a small but meaningful group of long-term responders can dramatically shift OS curves while having almost no impact on median PFS. The biology of ESCC produces long survival tails but minimal early radiographic change — breaking the trial-level relationship between PFS and OS.
Taken together with the low R² values and non-significant correlations in PD-L1–enriched subgroups, these findings indicate that PFS provides only weak trial-level surrogacy in ESCC and should not be used as a stand-alone primary endpoint.
These differences are central to interpreting the results, which were published as an open-access original research article in the European Journal of Cancer.
What This Means for Future Trials?
- In GEA, especially PD-L1–high (CPS ≥5) disease, PFS can be considered an adequate co-primary endpoint alongside OS.
- In PD-L1–low GEA, PFS loses predictive value, and OS remains essential for interpreting benefit.
- In ESCC, PFS does not capture the true magnitude of immunotherapy benefit, and current data support prioritizing OS as the key endpoint in future trials.
Conclusion
This meta-analysis provides important guidance for endpoint selection in first-line immunotherapy trials for gastroesophageal cancers. PFS is a useful co-primary surrogate endpoint in GEA, particularly in PD-L1–high disease where its correlation with OS is strong and biologically intuitive. In ESCC, however, PFS does not reflect long-term outcomes and should not replace OS as the primary measure of benefit.
Read full article here
You can read about KEYNOTE-859 Trial: HRQoL with 1L Pembrolizumab + Chemo in Advanced HER2-Negative Gastric/GEJ Cancer on OncoDaily.


